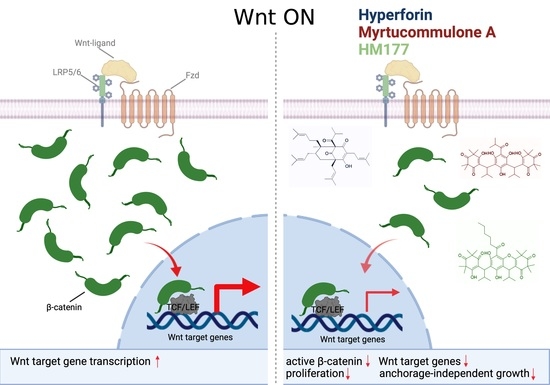
Hyperforin and Myrtucommulone Derivatives Act as Natural Modulators of Wnt/β-Catenin Signaling in HCT116 Colon Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. Cytotoxicity of HF and Myrtucommulones and Their Effects on Cell Viability
2.2. HF and HM 177 Exhibit Strong Anti-Proliferative Effect
2.3. MC A and HM 177 Strongly Inhibit Anchorage-Independent Growth
2.4. HF and MCs Repress Canonical Wnt Signaling
3. Discussion
4. Materials and Methods
4.1. Compounds
4.2. Cell Culture
4.3. Viability and Cytotoxicity Assays
4.4. Cell Proliferation and Anchorage-Independent Growth Assays
4.5. Reporter Gene Assays
4.6. Western Blot Analyses
4.7. Quantitative RT-PCR
4.8. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B

References
- Alipour, G.; Dashti, S.; Hosseinzadeh, H. Review of pharmacological effects of Myrtus communis L. and its active constituents. Phytother. Res. 2014, 28, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.A.; Martinez-Poveda, B.; Amores-Sanchez, M.I.; Quesada, A.R. Hyperforin: More than an antidepressant bioactive compound? Life Sci. 2006, 79, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Tatsis, E.C.; Boeren, S.; Exarchou, V.; Troganis, A.N.; Vervoort, J.; Gerothanassis, I.P. Identification of the major constituents of Hypericum perforatum by LC/SPE/NMR and/or LC/MS. Phytochemistry 2007, 68, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Napoli, E.; Siracusa, L.; Ruberto, G.; Carrubba, A.; Lazzara, S.; Speciale, A.; Cimino, F.; Saija, A.; Cristani, M. Phytochemical profiles, phototoxic and antioxidant properties of eleven Hypericum species—A comparative study. Phytochemistry 2018, 152, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Zirak, N.; Shafiee, M.; Soltani, G.; Mirzaei, M.; Sahebkar, A. Hypericum perforatum in the treatment of psychiatric and neurodegenerative disorders: Current evidence and potential mechanisms of action. J. Cell Physiol. 2019, 234, 8496–8508. [Google Scholar] [CrossRef] [PubMed]
- Biber, A.; Fischer, H.; Romer, A.; Chatterjee, S.S. Oral bioavailability of hyperforin from hypericum extracts in rats and human volunteers. Pharmacopsychiatry 1998, 31 (Suppl. S1), 36–43. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, R.; Salvatore, M.M.; Ferranti, P.; Andolfi, A. Structures and bioactive properties of myrtucommulones and related acylphloroglucinols from Myrtaceae. Molecules 2018, 23, 3370. [Google Scholar] [CrossRef] [Green Version]
- Rotstein, A.; Lifshitz, A.; Kashman, Y. Isolation and antibacterial activity of acylphloroglucinols from Myrtus communis. Antimicrob. Agents Chemother. 1974, 6, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Allegra, A.; Tonacci, A.; Spagnolo, E.V.; Musolino, C.; Gangemi, S. Antiproliferative effects of St. John’s wort, its derivatives, and other Hypericum species in hematologic malignancies. Int. J. Mol. Sci. 2021, 22, 146. [Google Scholar] [CrossRef] [PubMed]
- Menegazzi, M.; Masiello, P.; Novelli, M. Anti-tumor activity of Hypericum perforatum L. and hyperforin through modulation of inflammatory signaling, ROS generation and proton dynamics. Antioxidants 2021, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Albert, D.; Zundorf, I.; Dingermann, T.; Muller, W.E.; Steinhilber, D.; Werz, O. Hyperforin is a dual inhibitor of cyclooxygenase-1 and 5-lipoxygenase. Biochem. Pharmacol. 2002, 64, 1767–1775. [Google Scholar] [CrossRef]
- Feisst, C.; Pergola, C.; Rakonjac, M.; Rossi, A.; Koeberle, A.; Dodt, G.; Hoffmann, M.; Hoernig, C.; Fischer, L.; Steinhilber, D.; et al. Hyperforin is a novel type of 5-lipoxygenase inhibitor with high efficacy in vivo. Cell Mol. Life Sci. 2009, 66, 2759–2771. [Google Scholar] [CrossRef]
- Koeberle, A.; Rossi, A.; Bauer, J.; Dehm, F.; Verotta, L.; Northoff, H.; Sautebin, L.; Werz, O. Hyperforin, an anti-inflammatory constituent from St. John’s wort, inhibits microsomal prostaglandin E-2 synthase-1 and suppresses prostaglandin E-2 formation in vivo. Front. Pharmacol. 2011, 2, 7. [Google Scholar] [CrossRef] [Green Version]
- Hammer, K.D.P.; Hillwig, M.L.; Solco, A.K.; Dixon, P.M.; Delate, K.; Murphy, P.A.; Wurtele, E.S.; Birt, D.F. Inhibition of prostaglandin E(2) production by anti-inflammatory hypericum perforatum extracts and constituents in RAW264.7 Mouse Macrophage Cells. J. Agric. Food Chem. 2007, 55, 7323–7331. [Google Scholar] [CrossRef] [Green Version]
- Novelli, M.; Masiello, P.; Beffy, P.; Menegazzi, M. Protective role of St. John’s wort and its components hyperforin and hypericin against diabetes through inhibition of inflammatory signaling: Evidence from in vitro and in vivo studies. Int. J. Mol. Sci. 2020, 21, 8108. [Google Scholar] [CrossRef]
- Feisst, C.; Franke, L.; Appendino, G.; Werz, O. Identification of molecular targets of the oligomeric nonprenylated acylphloroglucinols from Myrtus communis and their implication as anti-inflammatory compounds. J. Pharmacol. Exp. Ther. 2005, 315, 389–396. [Google Scholar] [CrossRef] [Green Version]
- Gerbeth, K.; Husch, J.; Meins, J.; Rossi, A.; Sautebin, L.; Wiechmann, K.; Werz, O.; Skarke, C.; Barrett, J.S.; Schubert-Zsilavecz, M.; et al. Myrtucommulone from Myrtus communis: Metabolism, permeability, and systemic exposure in rats. Planta. Med. 2012, 78, 1932–1938. [Google Scholar] [CrossRef] [Green Version]
- Koeberle, A.; Pollastro, F.; Northoff, H.; Werz, O. Myrtucommulone, a natural acylphloroglucinol, inhibits microsomal prostaglandin E(2) synthase-1. Br. J. Pharmacol. 2009, 156, 952–961. [Google Scholar] [CrossRef] [Green Version]
- Wiechmann, K.; Muller, H.; Huch, V.; Hartmann, D.; Werz, O.; Jauch, J. Synthesis and biological evaluation of novel myrtucommulones and structural analogues that target mPGES-1 and 5-lipoxygenase. Eur. J. Med. Chem. 2015, 101, 133–149. [Google Scholar] [CrossRef]
- Grandjenette, C.; Schnekenburger, M.; Morceau, F.; Mack, F.; Wiechmann, K.; Werz, O.; Dicato, M.; Diederich, M. Dual induction of mitochondrial apoptosis and senescence in chronic myelogenous leukemia by myrtucommulone A. Anticancer Agents Med. Chem. 2015, 15, 363–373. [Google Scholar] [CrossRef]
- Wiechmann, K.; Muller, H.; Konig, S.; Wielsch, N.; Svatos, A.; Jauch, J.; Werz, O. Mitochondrial chaperonin Hsp60 is the apoptosis-related target for myrtucommulone. Cell Chem. Biol. 2017, 24, 614–623.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.C.; Lin, K.H.; Hsieh, J.H.; Chung, J.G.; Tan, Z.L.; Hsu, F.T.; Chiang, C.H. Hyperforin induces apoptosis through extrinsic/intrinsic pathways and inhibits NF-kB-modulated survival and invasion potential in bladder cancer. In Vivo 2019, 33, 1865–1877. [Google Scholar] [CrossRef] [PubMed]
- Izgi, K.; Iskender, B.; Jauch, J.; Sezen, S.; Cakir, M.; Charpentier, M.; Canatan, H.; Sakalar, C. Myrtucommulone-A induces both extrinsic and intrinsic apoptotic pathways in cancer cells. J. Biochem. Mol. Toxicol. 2015, 29, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Hostanska, K.; Reichling, J.; Bommer, S.; Weber, M.; Saller, R. Hyperforin a constituent of St John’s wort (Hypericum perforatum L.) extract induces apoptosis by triggering activation of caspases and with hypericin synergistically exerts cytotoxicity towards human malignant cell lines. Eur. J. Pharm. Biopharm. 2003, 56, 121–132. [Google Scholar] [CrossRef]
- Schempp, C.M.; Kirkin, V.; Simon-Haarhaus, B.; Kersten, A.; Kiss, J.; Termeer, C.C.; Gilb, B.; Kaufmann, T.; Borner, C.; Sleeman, J.P.; et al. Inhibition of tumour cell growth by hyperforin, a novel anticancer drug from St. John’s wort that acts by induction of apoptosis. Oncogene 2002, 21, 1242–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dona, M.; Dell’Aica, I.; Pezzato, E.; Sartor, L.; Calabrese, F.; Della Barbera, M.; Donella-Deana, A.; Appendino, G.; Borsarini, A.; Caniato, R.; et al. Hyperforin inhibits cancer invasion and metastasis. Cancer Res. 2004, 64, 6225–6232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iskender, B.; Izgi, K.; Karaca, H.; Canatan, H. Myrtucommulone-A treatment decreases pluripotency- and multipotency-associated marker expression in bladder cancer cell line HTB-9. J. Nat. Med. 2015, 69, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Imreova, P.; Feruszova, J.; Kyzek, S.; Bodnarova, K.; Zduriencikova, M.; Kozics, K.; Mucaji, P.; Galova, E.; Sevcovicova, A.; Miadokova, E.; et al. Hyperforin exhibits antigenotoxic activity on human and bacterial cells. Molecules 2017, 22, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satia, J.A.; Littman, A.; Slatore, C.G.; Galanko, J.A.; White, E. Associations of herbal and specialty supplements with lung and colorectal cancer risk in the VITamins and Lifestyle study. Cancer Epidem. Biomar. 2009, 18, 1419–1428. [Google Scholar] [CrossRef] [Green Version]
- Manna, S.K.; Golla, S.; Golla, J.P.; Tanaka, N.; Cai, Y.; Takahashi, S.; Krausz, K.W.; Matsubara, T.; Korboukh, I.; Gonzalez, F.J. St. John’s Wort attenuates colorectal carcinogenesis in mice through suppression of inflammatory signaling. Cancer Prev. Res. 2015, 8, 786–795. [Google Scholar] [CrossRef] [Green Version]
- Karaarslan, S.; Cokmert, S.; Cokmez, A. Does St. John’s Wort cause regression in gastrointestinal system adenocarcinomas? World J. Gastro. Oncol. 2015, 7, 369–374. [Google Scholar] [CrossRef]
- Clevers, H.; Nusse, R. Wnt/-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef] [Green Version]
- Caspi, M.; Wittenstein, A.; Kazelnik, M.; Shor-Nareznoy, Y.; Rosin-Arbesfeld, R. Therapeutic targeting of the oncogenic Wnt signaling pathway for treating colorectal cancer and other colonic disorders. Adv. Drug Deliv. Rev. 2021, 169, 118–136. [Google Scholar] [CrossRef]
- Jaiswal, A.S.; Marlow, B.P.; Gupta, N.; Narayan, S. β-Catenin-mediated transactivation and cell-cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene 2002, 21, 8414–8427. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, N.N.; Carothers, A.M.; Grunberger, D.; Bilinski, R.T.; Churchill, M.R.; Martucci, C.; Newmark, H.L.; Bertagnolli, M.M. Plant phenolics decrease intestinal tumors in an animal model of familial adenomatous polyposis. Carcinogenesis 2000, 21, 921–927. [Google Scholar] [CrossRef] [Green Version]
- Albring, K.F.; Weidemuller, J.; Mittag, S.; Weiske, J.; Friedrich, K.; Geroni, M.C.; Lombardi, P.; Huber, O. Berberine acts as a natural inhibitor of Wnt/β-catenin signaling--identification of more active 13-arylalkyl derivatives. Biofactors 2013, 39, 652–662. [Google Scholar] [CrossRef]
- Wu, K.; Yang, Q.; Mu, Y.; Zhou, L.; Liu, Y.; Zhou, Q.; He, B. Berberine inhibits the proliferation of colon cancer cells by inactivating Wnt/β-catenin signaling. Int. J. Oncol. 2012, 41, 292–298. [Google Scholar] [CrossRef] [Green Version]
- Teiten, M.H.; Gaascht, F.; Dicato, M.; Diederich, M. Targeting the wingless signaling pathway with natural compounds as chemopreventive or chemotherapeutic agents. Curr. Pharm. Biotechnol. 2012, 13, 245–254. [Google Scholar] [CrossRef]
- Wiechmann, K.; Muller, H.; Fischer, D.; Jauch, J.; Werz, O. The acylphloroglucinols hyperforin and myrtucommulone A cause mitochondrial dysfunctions in leukemic cells by direct interference with mitochondria. Apoptosis 2015, 20, 1508–1517. [Google Scholar] [CrossRef]
- Li, C.; Song, G.; Zhang, S.; Wang, E.; Cui, Z. Wnt3a increases the metastatic potential of non-small cell lung cancer cells in vitro in part via its upregulation of Notch3. Oncol. Rep. 2015, 33, 1207–1214. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Wang, Y.; Dabdoub, A.; Smallwood, P.M.; Williams, J.; Woods, C.; Kelley, M.W.; Jiang, L.; Tasman, W.; Zhang, K.; et al. Vascular development in the retina and inner ear: Control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell 2004, 116, 883–895. [Google Scholar] [CrossRef] [Green Version]
- Jho, E.H.; Zhang, T.; Domon, C.; Joo, C.K.; Freund, J.N.; Costantini, F. Wnt/β-catenin/TCF signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol. Cell Biol. 2002, 22, 1172–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lustig, B.; Jerchow, B.; Sachs, M.; Weiler, S.; Pietsch, T.; Karsten, U.; van de Wetering, M.; Clevers, H.; Schlag, P.M.; Birchmeier, W.; et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol. Cell. Biol. 2002, 22, 1184–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huggins, I.J.; Bos, T.; Gaylord, O.; Jessen, C.; Lonquich, B.; Puranen, A.; Richter, J.; Rossdam, C.; Brafman, D.; Gaasterland, T.; et al. The Wnt target SP5 negatively regulates Wnt transcriptional programs in human pluripotent stem cells. Nat. Commun. 2017, 8, 1034. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.W.; Chalamalasetty, R.B.; Thomas, S.; Garriock, R.J.; Jailwala, P.; Yamaguchi, T.P. Sp5 and Sp8 recruit β-catenin and Tcf1-Lef1 to select enhancers to activate Wnt target gene transcription. Proc. Natl. Acad. Sci. USA 2016, 113, 3545–3550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apaydin, E.A.; Maher, A.R.; Shanman, R.; Booth, M.S.; Miles, J.N.; Sorbero, M.E.; Hempel, S. A systematic review of St. John’s wort for major depressive disorder. Syst. Rev. 2016, 5, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merhi, F.; Tang, R.; Piedfer, M.; Mathieu, J.; Bombarda, I.; Zaher, M.; Kolb, J.P.; Billard, C.; Bauvois, B. Hyperforin inhibits Akt1 kinase activity and promotes caspase-mediated apoptosis involving Bad and Noxa activation in human myeloid tumor cells. PLoS ONE 2011, 6, e25963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, D.; Hawke, D.; Zheng, Y.; Xia, Y.; Meisenhelder, J.; Nika, H.; Mills, G.B.; Kobayashi, R.; Hunter, T.; Lu, Z. Phosphorylation of β-catenin by AKT promotes β-catenin transcriptional activity. J. Biol. Chem. 2007, 282, 11221–11229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iskender, B.; Izgi, K.; Canatan, H. Novel anti-cancer agent myrtucommulone-A and thymoquinone abrogate epithelial-mesenchymal transition in cancer cells mainly through the inhibition of PI3K/AKT signalling axis. Mol. Cell Biochem. 2016, 416, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Iskender, B.; Izgi, K.; Sakalar, C.; Canatan, H. Priming hMSCs with a putative anti-cancer compound, myrtucommulone-a: A way to harness hMSC cytokine expression via modulating PI3K/Akt pathway? Tumour. Biol. 2016, 37, 1967–1981. [Google Scholar] [CrossRef] [PubMed]
- Castellone, M.D.; Teramoto, H.; Williams, B.O.; Druey, K.M.; Gutkind, J.S. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-β-catenin signaling axis. Science 2005, 310, 1504–1510. [Google Scholar] [CrossRef]
- Traeger, A.; Voelker, S.; Shkodra-Pula, B.; Kretzer, C.; Schubert, S.; Gottschaldt, M.; Schubert, U.S.; Werz, O. Improved bioactivity of the natural product 5-lipoxygenase inhibitor hyperforin by encapsulation into polymeric nanoparticles. Mol. Pharm. 2020, 17, 810–816. [Google Scholar] [CrossRef]
- Müller, H.; Paul, M.; Hartmann, D.; Huch, V.; Blaesius, D.; Koeberle, A.; Werz, O.; Jauch, J. Total synthesis of myrtucommulone A. Angew. Chem. Int. Ed. Engl. 2010, 49, 2045–2049. [Google Scholar] [CrossRef]
- Appendino, G.; Bianchi, F.; Minassi, A.; Sterner, O.; Ballero, M.; Gibbons, S. Oligomeric acylphloroglucinols from myrtle (Myrtus communis). J. Nat. Prod. 2002, 65, 334–338. [Google Scholar] [CrossRef]
- Ye, Z.; Mittag, S.; Schmidt, M.; Simm, A.; Horstkorte, R.; Huber, O. Wnt Glycation Inhibits Canonical Signaling. Cells 2019, 8, 1320. [Google Scholar] [CrossRef] [Green Version]
- Weiske, J.; Albring, K.F.; Huber, O. The tumor suppressor Fhit acts as a repressor of β-catenin transcriptional activity. Proc. Natl. Acad. Sci. USA 2007, 104, 20344–20349. [Google Scholar] [CrossRef] [Green Version]
- Niyazi, M.; Niyazi, I.; Belka, C. Counting colonies of clonogenic assays by using densitometric software. Radiat. Oncol. 2007, 2, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Mittag, S.; Valenta, T.; Weiske, J.; Bloch, L.; Klingel, S.; Gradl, D.; Wetzel, F.; Chen, Y.; Petersen, I.; Basler, K.; et al. A novel role for the tumour suppressor Nitrilase1 modulating the Wnt/β-catenin signalling pathway. Cell Discov. 2016, 2, 15039. [Google Scholar] [CrossRef] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knauthe, A.; Mittag, S.; Bloch, L.; Albring, K.F.; Schmidt, M.; Werz, O.; Huber, O. Hyperforin and Myrtucommulone Derivatives Act as Natural Modulators of Wnt/β-Catenin Signaling in HCT116 Colon Cancer Cells. Int. J. Mol. Sci. 2022, 23, 2984. https://doi.org/10.3390/ijms23062984
Knauthe A, Mittag S, Bloch L, Albring KF, Schmidt M, Werz O, Huber O. Hyperforin and Myrtucommulone Derivatives Act as Natural Modulators of Wnt/β-Catenin Signaling in HCT116 Colon Cancer Cells. International Journal of Molecular Sciences. 2022; 23(6):2984. https://doi.org/10.3390/ijms23062984
Chicago/Turabian StyleKnauthe, Aneliya, Sonnhild Mittag, Laura Bloch, Kai Frederik Albring, Martin Schmidt, Oliver Werz, and Otmar Huber. 2022. "Hyperforin and Myrtucommulone Derivatives Act as Natural Modulators of Wnt/β-Catenin Signaling in HCT116 Colon Cancer Cells" International Journal of Molecular Sciences 23, no. 6: 2984. https://doi.org/10.3390/ijms23062984