Using Graphene-Based Materials for Stiff and Strong Poly(ethylene glycol) Hydrogels
Abstract
:1. Introduction
2. Results
2.1. FLGO and FLG Platelets Are Larger than GO
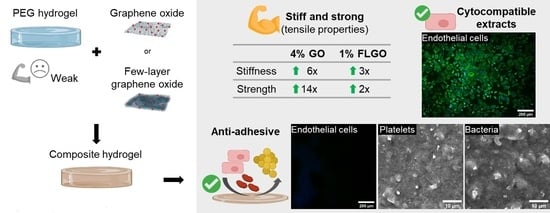
2.2. Oxidized GBM can Mechanically Reinforce PEG Hydrogels
2.3. PEG/GBM Composites Maintain Hydrophilicity of Neat PEG Hydrogels
2.4. Extracts of PEG/GBM Composite Hydrogels Are Cytocompatible towards HUVEC
2.5. Neither HUVEC, Platelets or Bacteria Adhere onto the Surface of PEG/GBM Composite Hydrogels
3. Discussion
4. Materials & Methods
4.1. Synthesis of GBM
4.2. Characterization of GBM
4.2.1. Transmission Electron Microscopy (TEM)
4.2.2. X-ray Photoelectron Spectroscopy (XPS)
4.3. Production of Neat PEG and PEG/GBM Composite Hydrogels
4.4. Mechanical Properties of Hydrogels
4.5. Physicochemical Properties of Hydrogels
4.5.1. Optical Microscopy
4.5.2. Scanning Electron Microscopy (SEM)
4.5.3. Optical Contact Angle (OCA)
4.5.4. Water Uptake Measurements
4.6. Cytocompatibility of Hydrogels’ Extracts
4.6.1. Cell Line and Culture Conditions
4.6.2. Preparation of Extracts
4.6.3. Extracts Assay
4.7. Anti-Adhesive Properties of Hydrogels
4.7.1. HUVEC Adhesion Assay
4.7.2. Platelet Adhesion Assay
4.7.3. Bacterial Adhesion Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mattingly, Q. Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases/#tab=tab_1 (accessed on 1 October 2021).
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef]
- Jaffer, I.H.; Weitz, J.I. The blood compatibility challenge. Part 1: Blood-contacting medical devices: The scope of the problem. Acta Biomater. 2019, 94, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Prosthetic Heart Valve/Artificial Heart Valve Market by Type (Transcatheter Heart Valve, Tissue Heart Valve, and Mechanical Heart Valve), and Region (North America, Europe, Asia-Pacific, and RoW)—Global Forecast to 2022; Markets and Markets: Northbrook, IL, USA, June 2017.
- Vascular Stent Market by Type (BMS, BVS, Drug Eluting), Product (Coronary, Peripheral, Carotid, Femoral, Aortic Aneurysm), Material [Metal (Stainless Steel, PtCr, Nitinol), Polymer], End User (Hospital, Cardiology Center, ASC)—Global Forecast to 2021; Markets and Markets: Northbrook, IL, USA, May 2017.
- Vascular Graft Market by Indication (EVAR, Abdominal Aneurysm Repair, Thoracic Aneurysm, Peripheral Vascular Repair), Raw Material (Polyester, ePTFE, Polyurethane, Biosynthetic), End Users (Hospital, Ambulatory Surgery Center)—Global Forecast to 2022; MD 5928; Markets and Markets: Northbrook, IL, USA, January 2018.
- Heart Pump Device Market by Product (Ventricular Assist Devices (LVAD, RVAD, BiVAD, and pVAD), Intra-Aortic Balloon Pumps, TAH), Type (Extracorporeal and Implantable Pumps), Therapy (Bridge-to-transplant, Destination Therapy)—Global Forecast to 2026; Markets and Markets: Northbrook, IL, USA, May 2021.
- Kapadia, M.R.; Popowich, D.A.; Kibbe, M.R. Modified Prosthetic Vascular Conduits. Circulation 2008, 117, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Post, S.; Kraus, T.; Müller-Reinartz, U.; Weiss, C.; Kortmann, H.; Quentmeier, A.; Winkler, M.; Husfeldt, K.J.; Allenberg, J.R. Dacron vs. Polytetrafluoroethylene Grafts for Femoropopliteal Bypass:a Prospective Randomised Multicentre Trial. Eur. J. Vasc. Endovasc. Surg. 2001, 22, 226–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dangas, G.D.; Weitz, J.I.; Giustino, G.; Makkar, R.; Mehran, R. Prosthetic Heart Valve Thrombosis. J. Am. Coll. Cardiol. 2016, 68, 2670–2689. [Google Scholar] [CrossRef]
- Lin, S.S.; Tiong, I.Y.H.; Asher, C.R.; Murphy, M.T.; Thomas, J.D.; Griffin, B.P. Prediction of thrombus-related mechanical prosthetic valve dysfunction using transesophageal echocardiography. Am. J. Cardiol. 2000, 86, 1097–1101. [Google Scholar] [CrossRef]
- Califano, S.; Pagani, F.D.; Malani, P.N. Left Ventricular Assist Device–Associated Infections. Infect. Dis. Clin. North Am. 2012, 26, 77–87. [Google Scholar] [CrossRef]
- Kirklin, J.K.; Naftel, D.C.; Kormos, R.L.; Stevenson, L.W.; Pagani, F.D.; Miller, M.A.; Ulisney, K.L.; Baldwin, J.T.; Young, J.B. Second INTERMACS annual report: More than 1,000 primary left ventricular assist device implants. J. Heart Lung. Transplant. 2010, 29, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Brash, J.L.; Horbett, T.A.; Latour, R.A.; Tengvall, P. The blood compatibility challenge. Part 2: Protein adsorption phenomena governing blood reactivity. Acta Biomater. 2019, 94, 11–24. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 54, 3–12. [Google Scholar] [CrossRef]
- Hoffmann, J.; Groll, J.; Heuts, J.; Rong, H.; Klee, D.; Ziemer, G.; Moeller, M.; Wendel, H.P. Blood cell and plasma protein repellent properties of Star-PEG-modified surfaces. J. Biomater. Sci. Polym. Ed. 2006, 17, 985–996. [Google Scholar] [CrossRef] [PubMed]
- Noel, S.; Hachem, A.; Merhi, Y.; De Crescenzo, G. Development of a polyester coating combining antithrombogenic and cell adhesive properties: Influence of sequence and surface density of adhesion peptides. Biomacromolecules 2015, 16, 1682–1694. [Google Scholar] [CrossRef]
- Maitz, M.F.; Martins, M.C.L.; Grabow, N.; Matschegewski, C.; Huang, N.; Chaikof, E.L.; Barbosa, M.A.; Werner, C.; Sperling, C. The blood compatibility challenge. Part 4: Surface modification for hemocompatible materials: Passive and active approaches to guide blood-material interactions. Acta Biomater. 2019, 94, 33–43. [Google Scholar] [CrossRef]
- Hahn, M.S.; McHale, M.K.; Wang, E.; Schmedlen, R.H.; West, J.L. Physiologic pulsatile flow bioreactor conditioning of poly(ethylene glycol)-based tissue engineered vascular grafts. Ann. Biomed. Eng. 2007, 35, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Bracaglia, L.G.; Messina, M.; Winston, S.; Kuo, C.-Y.; Lerman, M.; Fisher, J.P. 3D Printed Pericardium Hydrogels To Promote Wound Healing in Vascular Applications. Biomacromolecules 2017, 18, 3802–3811. [Google Scholar] [CrossRef]
- Wang, P.-H.; Lin, C.-H.; Wen, T.-C. Tough and antifouling polyampholyte hydrogels via photopolymerization of equivalent ionic monomers with poly(ethylene glycol) diacrylate. J. Taiwan Inst. Chem. Eng. 2020, 113, 101–106. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Henriques, P.C.; Borges, I.; Pinto, A.M.; Magalhães, F.D.; Gonçalves, I.C. Fabrication and antimicrobial performance of surfaces integrating graphene-based materials. Carbon 2018, 132, 709–732. [Google Scholar] [CrossRef]
- Pinto, A.M.; Goncąlves, C.; Sousa, D.M.; Ferreira, A.R.; Moreira, J.A.; Goncąlves, I.C.; Magalhães, F.D. Smaller particle size and higher oxidation improves biocompatibility of graphene-based materials. Carbon 2016, 99, 318–329. [Google Scholar] [CrossRef]
- Melo, S.F.; Neves, S.C.; Pereira, A.T.; Borges, I.; Granja, P.L.; Magalhães, F.D.; Gonçalves, I.C. Incorporation of graphene oxide into poly(ɛ-caprolactone) 3D printed fibrous scaffolds improves their antimicrobial properties. Mater. Sci. Eng. C 2020, 109, 110537. [Google Scholar] [CrossRef]
- Pereira, A.T.; Henriques, P.C.; Costa, P.C.; Martins, M.C.L.; Magalhães, F.D.; Gonçalves, I.C. Graphene oxide-reinforced poly(2-hydroxyethyl methacrylate) hydrogels with extreme stiffness and high-strength. Compos. Sci. Technol. 2019, 184, 107819. [Google Scholar] [CrossRef]
- Pereira, A.T.; Henriques, P.C.; Schneider, K.H.; Pires, A.L.; Pereira, A.M.; Martins, M.C.L.; Magalhães, F.D.; Bergmeister, H.; Gonçalves, I.C. Graphene-based materials: The key for the successful application of pHEMA as a blood-contacting device. Biomater. Sci. 2021, 9, 3362–3377. [Google Scholar] [CrossRef]
- Teodorescu, F.; Oz, Y.; Quéniat, G.; Abderrahmani, A.; Foulon, C.; Lecoeur, M.; Sanyal, R.; Sanyal, A.; Boukherroub, R.; Szunerits, S. Photothermally triggered on-demand insulin release from reduced graphene oxide modified hydrogels. J. Control. Release 2017, 246, 164–173. [Google Scholar] [CrossRef]
- Jang, J.; Hong, J.; Cha, C. Effects of precursor composition and mode of crosslinking on mechanical properties of graphene oxide reinforced composite hydrogels. J. Mech. Behav. Biomed. Mater. 2017, 69, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Wu, G.; Zhou, H.; Qian, K.; Hu, J. Preparation and property evaluation of conductive hydrogel using poly (vinyl alcohol)/polyethylene glycol/graphene oxide for human electrocardiogram acquisition. Polymers 2017, 9, 259. [Google Scholar] [CrossRef]
- Ahadian, S.; Naito, U.; Surya, V.J.; Darvishi, S.; Estili, M.; Liang, X.; Nakajima, K.; Shiku, H.; Kawazoe, Y.; Matsue, T. Fabrication of poly(ethylene glycol) hydrogels containing vertically and horizontally aligned graphene using dielectrophoresis: An experimental and modeling study. Carbon 2017, 123, 460–470. [Google Scholar] [CrossRef]
- Bao, R.; Tan, B.; Liang, S.; Zhang, N.; Wang, W.; Liu, W. A π-π conjugation-containing soft and conductive injectable polymer hydrogel highly efficiently rebuilds cardiac function after myocardial infarction. Biomaterials 2017, 122, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.K.; Turner, E.E.; Chalfant, B.H.; Yadavalli, V.K. Mechanically robust, photopatternable conductive hydrogel composites. React. Funct. Polym. 2017, 120, 66–73. [Google Scholar] [CrossRef]
- Mamaghani, K.R.; Naghib, S.M.; Zahedi, A.; Rahmanian, M.; Mozafari, M. GelMa/PEGDA containing graphene oxide as an IPN hydrogel with superior mechanical performance. Mater. Today Proc. 2018, 5 (Pt 3), 15790–15799. [Google Scholar] [CrossRef]
- ASTM D882-18; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM: West Conshohocken, PA, USA, 2018.
- ISO 10993-12:2012; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. ISO: Geneva, Switzerland, 2012.
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009; p. 34.
- Cheryk, L.A.; Gentry, P.A.; Tablin, F. Morphological characteristics of bovine platelets activated with platelet activating factor or thrombin. Comp. Haematol. Int. 1997, 7, 88–94. [Google Scholar] [CrossRef]
- Iqbal, A.A.; Sakib, N.; Iqbal, A.K.M.P.; Nuruzzaman, D.M. Graphene-based nanocomposites and their fabrication, mechanical properties and applications. Materialia 2020, 12, 100815. [Google Scholar] [CrossRef]
- Han, L.; Lu, X.; Wang, M.; Gan, D.; Deng, W.; Wang, K.; Fang, L.; Liu, K.; Chan, C.W.; Tang, Y.; et al. A Mussel-Inspired Conductive, Self-Adhesive, and Self-Healable Tough Hydrogel as Cell Stimulators and Implantable Bioelectronics. Small 2017, 13, 1601916. [Google Scholar] [CrossRef]
- Hu, K.; Kulkarni, D.D.; Choi, I.; Tsukruk, V.V. Graphene-polymer nanocomposites for structural and functional applications. Prog. Polym. Sci. 2014, 39, 1934–1972. [Google Scholar] [CrossRef]
- Parlato, M.; Reichert, S.; Barney, N.; Murphy, W.L. Poly(ethylene glycol) hydrogels with adaptable mechanical and degradation properties for use in biomedical applications. Macromol. Biosci. 2014, 14, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hong, W.; Bai, H.; Li, C.; Shi, G. Strong and ductile poly(vinyl alcohol)/graphene oxide composite films with a layered structure. Carbon 2009, 47, 3538–3543. [Google Scholar] [CrossRef]
- Van Den Broeck, L.; Piluso, S.; Soultan, A.H.; De Volder, M.; Patterson, J. Cytocompatible carbon nanotube reinforced polyethylene glycol composite hydrogels for tissue engineering. Mater. Sci. Eng. C 2019, 98, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Ragaert, K.; Swieszkowski, W.; Selimović, Š.; Paul, A.; Camci-Unal, G.; Mofrad, M.R.K.; Khademhosseini, A. Biomechanical properties of native and tissue engineered heart valve constructs. J. Biomech. 2014, 47, 1949–1963. [Google Scholar] [CrossRef]
- Leeson-Dietrich, J.; Boughner, D.; Vesely, I. Porcine pulmonary and aortic valves: A comparison of their tensile viscoelastic properties at physiological strain rates. J. Heart Valve Dis. 1995, 4, 88–94. [Google Scholar]
- Christie, G.W. Anatomy of aortic heart valve leaflets: The influence of glutaraldehyde fixation on function. Eur. J. Cardio Thorac. Surg. 1992, 6, S25–S33. [Google Scholar] [CrossRef]
- Claes, E.; Atienza, J.M.; Guinea, G.V.; Rojo, F.J.; Bernal, J.M.; Revuelta, J.M.; Elices, M. Mechanical properties of human coronary arteries. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 3792–3795. [Google Scholar]
- Chamiot-Clerc, P.; Copie, X.; Renaud, J.F.; Safar, M.; Girerd, X. Comparative reactivity and mechanical properties of human isolated internal mammary and radial arteries. Cardiovasc. Res. 1998, 37, 811–819. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, S.; Benoit, D.S.W. Degradable poly(ethylene glycol) (PEG)-based hydrogels for spatiotemporal control of siRNA/nanoparticle delivery. J. Control. Release 2018, 287, 58–66. [Google Scholar] [CrossRef]
- Wu, J.; Chen, A.; Qin, M.; Huang, R.; Zhang, G.; Xue, B.; Wei, J.; Li, Y.; Cao, Y.; Wang, W. Hierarchical construction of a mechanically stable peptide–graphene oxide hybrid hydrogel for drug delivery and pulsatile triggered release in vivo. Nanoscale 2015, 7, 1655–1660. [Google Scholar] [CrossRef] [PubMed]
- Valentin, T.M.; Landauer, A.K.; Morales, L.C.; DuBois, E.M.; Shukla, S.; Liu, M.; Stephens Valentin, L.H.; Franck, C.; Chen, P.-Y.; Wong, I.Y. Alginate-graphene oxide hydrogels with enhanced ionic tunability and chemomechanical stability for light-directed 3D printing. Carbon 2019, 143, 447–456. [Google Scholar] [CrossRef]
- Henriques, P.C.; Pereira, A.T.; Pires, A.L.; Pereira, A.M.; Magalhães, F.D.; Gonçalves, I.C. Graphene Surfaces Interaction with Bacteria, Mammalian Cells and Blood Constituents: The Impact of Graphene Platelets Oxidation and Thickness. ACS Appl. Mater. Interfaces 2020, 12, 21020–21035. [Google Scholar] [CrossRef]
- Wallitt, E.J.W.; Jevon, M.; Hornick, P.I. Therapeutics of Vein Graft Intimal Hyperplasia: 100 Years On. Ann. Thorac. Surg. 2007, 84, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Leslie-Barbick, J.E.; Saik, J.E.; Gould, D.J.; Dickinson, M.E.; West, J.L. The promotion of microvasculature formation in poly(ethylene glycol) diacrylate hydrogels by an immobilized VEGF-mimetic peptide. Biomaterials 2011, 32, 5782–5789. [Google Scholar] [CrossRef]
- Fonseca, K.B.; Granja, P.L.; Barrias, C.C. Engineering proteolytically-degradable artificial extracellular matrices. Prog. Polym. Sci. 2014, 39, 2010–2029. [Google Scholar] [CrossRef]
- Kovtun, A.; Jones, D.; Dell’Elce, S.; Treossi, E.; Liscio, A.; Palermo, V. Accurate chemical analysis of oxygenated graphene-based materials using X-ray photoelectron spectroscopy. Carbon 2019, 143, 268–275. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, H.P.; Moura, D.; Pereira, A.T.; Henriques, P.C.; Barrias, C.C.; Magalhães, F.D.; Gonçalves, I.C. Using Graphene-Based Materials for Stiff and Strong Poly(ethylene glycol) Hydrogels. Int. J. Mol. Sci. 2022, 23, 2312. https://doi.org/10.3390/ijms23042312
Ferreira HP, Moura D, Pereira AT, Henriques PC, Barrias CC, Magalhães FD, Gonçalves IC. Using Graphene-Based Materials for Stiff and Strong Poly(ethylene glycol) Hydrogels. International Journal of Molecular Sciences. 2022; 23(4):2312. https://doi.org/10.3390/ijms23042312
Chicago/Turabian StyleFerreira, Helena P., Duarte Moura, Andreia T. Pereira, Patrícia C. Henriques, Cristina C. Barrias, Fernão D. Magalhães, and Inês C. Gonçalves. 2022. "Using Graphene-Based Materials for Stiff and Strong Poly(ethylene glycol) Hydrogels" International Journal of Molecular Sciences 23, no. 4: 2312. https://doi.org/10.3390/ijms23042312