Evaluation of Cell Migration and Cytokines Expression Changes under the Radiofrequency Electromagnetic Field on Wound Healing In Vitro Model
Abstract
:1. Introduction
2. Results
2.1. RF-EMF Exposure Does Not Change the Viability of HaCaT Cells
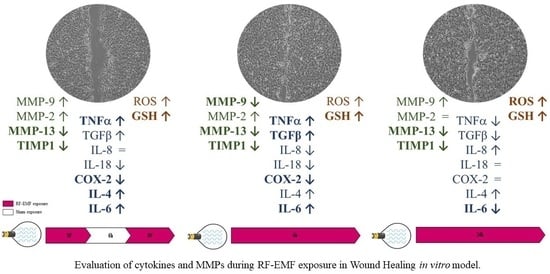
2.2. RF-EMF Modulates Oxidative Balance in HaCaT Cells
2.3. RF-EMF Promotes Proliferation and Migration of HaCaT Cells in a Model of Wound Healing
2.4. RF-EMF Modulates Expression of Inflammatory Mediators in HaCaT Cells
2.5. RF-EMF Modulates Expression and Production of IL-6
2.6. RF-EMF Modulates Expression of MMPs in HaCaT Cells
3. Discussion and Conclusions
4. Materials and Methods
4.1. Cell Culture
4.2. RF-EMF Exposure System
4.3. Experimental Protocols
4.4. Cell Proliferation Assay
4.5. Oxidative Stress
4.5.1. GSH Assay
4.5.2. ROS Assay
4.6. Mechanical Stretch Injury and Migration Rate Assays
4.7. Cell Proliferation Assay in Presence of Mitomycin C and Mechanical Scratch
4.8. Real-Time PCR
4.9. IL-6 Enzyme-Linked Immunosorbent Assay
5. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caley, M.P.; Martins, V.L.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serra, F.; Aielli, L.; Costantini, E. The role of miRNAs in the inflammatory phase of skin wound healing. AIMS Allergy Immunol. 2021, 5, 264–278. [Google Scholar] [CrossRef]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of Acute and Chronic Wound Healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef]
- Patruno, A.; Ferrone, A.; Costantini, E.; Franceschelli, S.; Pesce, M.; Speranza, L.; Amerio, P.; D’Angelo, C.; Felaco, M.; Grilli, A.; et al. Extremely low-frequency electromagnetic fields accelerates wound healing modulating MMP-9 and inflammatory cytokines. Cell Prolif. 2018, 51, e12432. [Google Scholar] [CrossRef] [Green Version]
- Bassett, C.A. Fundamental and practical aspects of therapeutic uses of pulsed electromagnetic fields (PEMFs). Crit. Rev. Biomed. Eng. 1989, 17, 451–529. [Google Scholar]
- Guo, L.; Kubat, N.J.; Nelson, T.R.; Isenberg, R.A. Meta-analysis of clinical efficacy of pulsed radiofrequency energy treatment. Ann. Surg. 2012, 255, 457–467. [Google Scholar] [CrossRef]
- Maier, M. Pulsed radio frequency energy in the treatment of painful chronic cutaneous wounds: A report of two cases. Pain Med. 2011, 12, 829–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piipponen, M.; Li, D.; Landén, N.X. The Immune Functions of Keratinocytes in Skin Wound Healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef] [PubMed]
- Widgerow, A.D. Chronic wound fluid—thinking outside the box. Wound Repair Regen. 2011, 19, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Toriseva, M.; Laato, M.; Carpén, O.; Ruohonen, S.T.; Savontaus, E.; Inada, M.; Krane, S.M.; Kähäri, V.-M. MMP-13 regulates growth of wound granulation tissue and modulates gene expression signatures involved in inflammation, proteolysis, and cell viability. PLoS ONE 2012, 7, e42596. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.C.; Costa, T.F.; Andrade, Z.A.; Medrado, A.R. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastar, I.; Stojadinovic, O.; Tomic-Canic, M. Role of keratinocytes in healing of chronic wounds. Surg. Technol. Int. 2008, 17, 105–112. [Google Scholar] [PubMed]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Brasier, A.R. The nuclear factor-kappaB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res. 2010, 86, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Johnson, B.Z.; Stevenson, A.W.; Prêle, C.M.; Fear, M.W.; Wood, F.M. The Role of IL-6 in Skin Fibrosis and Cutaneous Wound Healing. Biomedicines 2020, 8, 101. [Google Scholar] [CrossRef]
- Gallucci, R.M.; Sloan, D.K.; Heck, J.M.; Murray, A.R.; O’Dell, S.J. Interleukin 6 indirectly induces keratinocyte migration. J. Invest. Dermatol. 2004, 122, 764–772. [Google Scholar] [CrossRef] [Green Version]
- Rohani, M.G.; Parks, W.C. Matrix remodeling by MMPs during wound repair. Matrix Biol. 2015, 44–46, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Salonurmi, T.; Parikka, M.; Kontusaari, S.; Pirilä, E.; Munaut, C.; Salo, T.; Tryggvason, K. Overexpression of TIMP-1 under the MMP-9 promoter interferes with wound healing in transgenic mice. Cell Tissue Res. 2004, 315, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Gualdi, G.; Costantini, E.; Reale, M.; Amerio, P. Wound Repair and Extremely Low Frequency-Electromagnetic Field: Insight from In Vitro Study and Potential Clinical Application. Int. J. Mol. Sci. 2021, 22, 5037. [Google Scholar] [CrossRef] [PubMed]
- Patruno, A.; Pesce, M.; Grilli, A.; Speranza, L.; Franceschelli, S.; De Lutiis, M.A.; Vianale, G.; Costantini, E.; Amerio, P.; Muraro, R.; et al. mTOR Activation by PI3K/Akt and ERK Signaling in Short ELF-EMF Exposed Human Keratinocytes. PLoS ONE 2015, 10, e0139644. [Google Scholar] [CrossRef] [Green Version]
- Mangiacasale, R.; Tritarelli, A.; Sciamanna, I.; Cannone, M.; Lavia, P.; Barberis, M.C.; Lorenzini, R.; Cundari, E. Normal and cancer-prone human cells respond differently to extremely low frequency magnetic fields. FEBS Lett. 2001, 487, 397–403. [Google Scholar] [CrossRef] [Green Version]
- Cañedo-Dorantes, L.; García-Cantú, R.; Barrera, R.; Méndez-Ramírez, I.; Navarro, V.H.; Serrano, G. Healing of chronic arterial and venous leg ulcers through systemic effects of electromagnetic fields [corrected]. Arch. Med. Res. 2002, 33, 281–289, Erratum in: Arch. Med. Res. 2002, 33, 513. [Google Scholar] [CrossRef]
- Kwan, R.L.; Lu, S.; Choi, H.M.; Kloth, L.C.; Cheing, G.L. Efficacy of Biophysical Energies on Healing of Diabetic Skin Wounds in Cell Studies and Animal Experimental Models: A Systematic Review. Int. J. Mol. Sci. 2019, 20, 368. [Google Scholar] [CrossRef] [Green Version]
- Zeni, O.; Romeo, S.; Sannino, A.; Palumbo, R.; Scarfì, M.R. Evidence of bystander effect induced by radiofrequency radiation in a human neuroblastoma cell line. Environ. Res. 2021, 196, 110935. [Google Scholar] [CrossRef]
- Kwan, R.L.; Wong, W.C.; Yip, S.L.; Chan, K.L.; Zheng, Y.P.; Cheing, G.L. Pulsed electromagnetic field therapy promotes healing and microcirculation of chronic diabetic foot ulcers: A pilot study. Adv. Skin Wound Care 2015, 28, 212–219. [Google Scholar] [CrossRef]
- Glassman, L.S.; McGrath, M.H.; Bassett, C.A. Effect of external pulsing electromagnetic fields on the healing of soft tissue. Ann. Plast. Surg. 1986, 16, 287–295. [Google Scholar] [CrossRef]
- Rawe, I.M.; Lowenstein, A.; Barcelo, C.R.; Genecov, D.G. Control of postoperative pain with a wearable continuously operating pulsed radiofrequency energy device: A preliminary study. Aesthetic Plast. Surg. 2012, 36, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Li, F.; Singh, T.P.; Wolf, P.; Wang, X.J. The pro-inflammatory role of TGFβ1: A paradox? Int. J. Biol. Sci. 2012, 8, 228–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, L.; Levine, A.D. TGF-beta inhibits IL-2 production and promotes cell cycle arrest in TCR-activated effector/memory T cells in the presence of sustained TCR signal transduction. J. Immunol. 2008, 180, 1490–1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, H.W.; Kim, I.S. TGF-beta1 enhances betaig-h3-mediated keratinocyte cell migration through the alpha3beta1 integrin and PI3K. J. Cell. Biochem. 2004, 92, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Aleem, S.A.E.R.; Jude, E. Inhibition of Wound TGF Beta-1 by Celecoxib: A Possible Therapeutic Route for Scar Free Wound. J. Cytol. Histol. 2017, 8, 481. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.G.; Felix, F.N.; Woodley, D.T.; Shim, E.K. The role of oxygen in wound healing: A review of the literature. Dermatol. Surg. 2008, 34, 1159–1169. [Google Scholar] [CrossRef]
- Schäfer, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef]
- Ponugoti, B.; Xu, F.; Zhang, C.; Tian, C.; Pacios, S.; Graves, D.T. FOXO1 promotes wound healing through the up-regulation of TGF-β1 and prevention of oxidative stress. J. Cell. Biol. 2013, 203, 327–343. [Google Scholar] [CrossRef] [Green Version]
- Moseley, R.; Stewart, J.E.; Stephens, P.; Waddington, R.J.; Thomas, D.W. Extracellular matrix metabolites as potential biomarkers of disease activity in wound fluid: Lessons learned from other inflammatory diseases? Br. J. Dermatol. 2004, 150, 401–413. [Google Scholar] [CrossRef]
- Costantini, E.; Sinjari, B.; D’Angelo, C.; Murmura, G.; Reale, M.; Caputi, S. Human Gingival Fibroblasts Exposed to Extremely Low-Frequency Electromagnetic Fields: In Vitro Model of Wound-Healing Improvement. Int. J. Mol. Sci. 2019, 20, 2108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reale, M.; D’Angelo, C.; Costantini, E.; Tata, A.M.; Regen, F.; Hellmann-Regen, J. Effect of Environmental Extremely Low-Frequency Electromagnetic Fields Exposure on Inflammatory Mediators and Serotonin Metabolism in a Human Neuroblastoma Cell Line. CNS Neurol. Disord. Drug Targets 2016, 15, 1203–1215. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef] [Green Version]
- Boulware, M.J.; Marchant, J.S. Timing in cellular Ca2+ signaling. Curr Biol. 2008, 18, R769–R776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]







| Gene | Forward Primer Sequence (5’-3’) | Reverse Primer Sequence (5’-3’) | Amplicon Lenght |
|---|---|---|---|
| TNFα | CCTTCCTGATCGTGGCAG | GCTTGAGGGTTTGCTACAAC | 184 bp |
| TGFβ | AACAATTCCTGGCGATACCTC | GTAGTGAACCCGTTGATGTCC | 197 bp |
| IL-8 | GTGTAAACATGACTTCCAAGCTG | GTCCACTCTCAATCACTCTCAG | 182 bp |
| IL-18 | CAGTCAGCAAGGAATTGTCTC | GAGGAAGCGATCTGGAAGG | 139 bp |
| COX-2 | GACAGTCCACCAACTTACAATG | GGCAATCATCAGGCACAGG | 105 bp |
| IL-4 | CAAGTGACTGACAATCTGGTG | AGTGACAATGTGAGGCAATTAG | 182 bp |
| IL-6 | GTACATCCTCGACGGCATC | ACCTCAAACTCCAAAAGACCAG | 198 bp |
| TIMP1 | CCAAGCCTTAGGGGATGCCG | GCTGTTCCAGGGAGCCACAA | 175 bp |
| MMP-9 | GTCTTCCCCTTCACTTTCCTG | GAGGAATGATCTAAGCCCAGC | 197 bp |
| MMP-2 | CAGTGACGGAAAGATGTGGT | TGGTGTAGGTGTAAATGGGTG | 182 bp |
| MMP-13 | GTTGCTGCGCATGAGTTCGG | AACCTGCTGAGGATGCAGGC | 294 bp |
| RPS18 | CTTTGCCATCACTGCCATTAAG | TCCATCCTTTACATCCTTCTGTC | 199 bp |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costantini, E.; Aielli, L.; Serra, F.; De Dominicis, L.; Falasca, K.; Di Giovanni, P.; Reale, M. Evaluation of Cell Migration and Cytokines Expression Changes under the Radiofrequency Electromagnetic Field on Wound Healing In Vitro Model. Int. J. Mol. Sci. 2022, 23, 2205. https://doi.org/10.3390/ijms23042205
Costantini E, Aielli L, Serra F, De Dominicis L, Falasca K, Di Giovanni P, Reale M. Evaluation of Cell Migration and Cytokines Expression Changes under the Radiofrequency Electromagnetic Field on Wound Healing In Vitro Model. International Journal of Molecular Sciences. 2022; 23(4):2205. https://doi.org/10.3390/ijms23042205
Chicago/Turabian StyleCostantini, Erica, Lisa Aielli, Federica Serra, Lorenzo De Dominicis, Katia Falasca, Pamela Di Giovanni, and Marcella Reale. 2022. "Evaluation of Cell Migration and Cytokines Expression Changes under the Radiofrequency Electromagnetic Field on Wound Healing In Vitro Model" International Journal of Molecular Sciences 23, no. 4: 2205. https://doi.org/10.3390/ijms23042205