Ten Approaches That Improve Immunostaining: A Review of the Latest Advances for the Optimization of Immunofluorescence
Abstract
:1. Introduction
- Sample preparation, related to the handling and maintenance of animals and cells under laboratory conditions;
- Replacement of blood (for whole animals) or serum media (from cells or isolated tissues) by saline buffers;
- Fixation, usually followed by a post-fixation step with an appropriate solution and temperature that vary depending on the tissue and organism type;
- Antigen retrieval;
- Permeabilization, sometimes as an independent step or carried out during the blocking stage, washing, or also applied during antibody incubations or nuclei counterstaining;
- Blocking of non-specific sites. In this step, reagents are generally applied before the primary antibody incubation, and it often is also used as the antibody’s incubation buffer and/or as rinse solution besides the blocking buffer;
- Primary antibody incubation. As mentioned above, primary antibodies are sometimes directly conjugated (direct method) or non-conjugated (indirect method);
- Secondary antibody incubation (indirect method);
- Washing (usually performed after fixation), antigen retrieval, and blocking incubation, as well as being followed by the incubation of primary and secondary antibodies;
- Nuclei counterstaining, usually a complementary step to immunostaining.
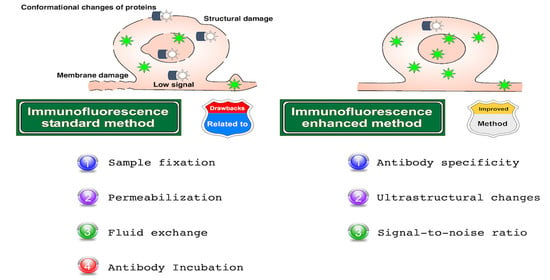
Drawbacks in Immunolabeling Methods
- Changes in protein localization as a consequence of: (a) protein alterations, (b) membrane damage, (c) loss or relocation of proteins, and (d) disruption of the native structure of tissues and cells;
- Changes in protein expression: as above;
- Hypoxia: (a) drastic decrease of oxygen levels;
- Phosphorylation: (a) protein alterations, (b) biochemical changes;
- Damage and cell death: (a) drastic decrease of oxygen levels, (b) loss of osmolarity, (c) biochemical changes, (d) loss of membrane surface tension, (e) membrane disruption (specially in cold fixation), and (f) disruption of the native structure of tissues and cells;
- Consequences related to osmolarity changes: (a) biochemical changes, (b) loss of membrane surface tension;
- Masking epitopes: (a) modifications of antigenic sites, (b) over-fixation;
- Background signal: (a) cell injury and cell death, (b) modifications of antigenic sites, (c) low signal-to-noise ratio, (d) fluctuations of protein structure, (e) autofluorescence, (f) low antibody signal, (g) unspecific binding, and (h) low antibody binding;
- Conformational changes of proteins: (a) protein alterations, (b) drastic decrease of oxygen levels, (c) biochemical changes, (d) cell injury and cell death, (e) modifications of antigenic sites, and (f) disruption of the native structure of tissues and cells;
- Protein loss: (a) loss of membrane surface tension, (b) membrane disruption (especially in cold fixation), (c) disruption of the native structure of tissues and cells;
- Membrane damage: (a) loss of membrane surface tension, (b) cell injury and cell death, and (c) disruption of the native structure of tissues and cells;
- Structural damage: (a) loss of osmolarity, loss of membrane surface tension, (b) cell injury and cell death, (c) membrane disruption (specially in cold fixation), and (d) disruption of the native structure of tissues and cells;
- Crosslinking interspecies serum: (a) use of sera and antibodies of similar species, (b) primary antibody raised in the same species as the sample;
- Low signal: (a) cell injury and cell death, (b) modifications of antigenic sites, (c) fluctuations of protein structure, (d) autofluorescence, (e) low penetration, or (f) low antibody binding;
- Poor penetration: (a) disruption of the native structure of tissues and cells, (b) poor permeabilization;
- Unspecific binding: (a) cell injury and cell death, (b) modifications of antigenic sites, (c) fluctuations of protein structure, and (d) crosslinking;
- Intense glare: (a) given by nuclear overstaining.
2. Methods to Improve Immunofluorescence
2.1. Improve Immunofluorescence by Applying Pretreatment with Triton X-100 in LR-White Thin Sections
2.1.1. Drawbacks to Face
2.1.2. Immunolabeling for Cell in Culture Embedded in LR-White and Pretreated with Triton X-100
- Collect and centrifuge cell suspension for 5 min *;
- Resuspend pellet in 5 mL of 0.1 M phosphate-buffered saline (PBS), pH 7.3;
- Centrifuge again;
- Wash cell pellets in PBS twice for 2 min;
- Before fixation, always pretreat with 0.2% Triton X-100 (Fisher Scientific) for 2 min;
- Fix with 4% formaldehyde (Fisher Scientific) and 0.2% glutaraldehyde (Sigma-Aldrich) for 15 min;
- Wash with PBS three times for 5 min each;
- Partially dehydrate cells with 30%, 50%, and 70% ethanol for 5 min each;
- Gradually infiltrate cells with LR-White (Canemco) using a ratio of 2:1 LR-White over 70% ethanol for 1 h on a rotary device;
- Infiltrate cells only with 100% LR-White twice for 1 h each with gentle agitation on a rotary device;
- Cold cure cells:
- a.
- Cool an accelerator to 0 °C and mix with LR-White resin;
- b.
- Place samples in gauge gelatin capsules (Canemco);
- c.
- Filled with LR-White-accelerator mixture.
- Place the sample-block in a refrigerator and leave to polymerize for 3 h at 0 °C;
- Cut sections using an ultramicrotome, transfer to poly-L-lysine pre-coated glass slides (Electron Microscopy Sciences) and leave to dry overnight;
- Block non-specific sites for 20 min in 3% bovine serum albumin (Sigma-Aldrich), 0.1% glycine (Electron Microscopy Sciences), 0.2% Tween 20 (Fisher Scientific), and 5% normal goat serum (Sigma Aldrich);
- Add primary antibody in blocking solution and incubate for 1 h;
- Rinse five times for 2 min each in PBS and block again for 20 min;
- Incubate with secondary antibody for 1 h;
- Rinse samples five times for 2 min in PBS and mount with Prolong antifade (Molecular Probes).
2.1.3. Key Points
2.1.4. Disadvantages
- (a)
- High cost of LR-White;
- (b)
- This technique can only be used when the antigen is resistant to 0.2% of Triton X-100 and;
- (c)
- Long-term Triton X-100 permeabilization induces cell membrane damage, as such do not apply for long periods at 0.2% or upper concentrations because even short times may increase fluorescence intensity, as has been previously reported when Triton X-100 viscosity is higher [37].
2.2. Application of Phosphatase Inhibitors to Improve Immunolocalization of Phosphorylated MAPKs
2.2.1. Drawbacks to Face
2.2.2. Tissue Preparation
- Perfuse transcardially using sterile physiological saline containing 1% (v/v) phosphatase inhibitor cocktail 2 (sodium orthovanadate, sodium molybdate, sodium tartrate, and imidazole; Sigma-Aldrich) and 0.01 M sodium fluoride;
- Proceed with fixative Davidson’s solution (formaldehyde, absolute ethanol, glacial acetic acid, and water (use the deionized or similar water that you use for your routine buffers); 2:3:1:3; this solution has been reported to reduce dephosphorylation of MAPK);
- Extract tissue and post-fix for 24 h by immersing in Davidson’s solution;
- Transfer to 70% (v/v) ethanol;
- Embed sections in paraffin;
- Cut 5 μm serial slices using a rotary microtome;
- Deparaffinize sections with xylene followed by 100% ethanol;
- Microwave for 10 min in boiling sodium citrate buffer (10 mM; pH 6.0) (antigen retrieval). For power of microwave, you can review protocol 2.6, Step 7, for frozen sections and Protocols 2.6 and 2.10, Steps 4 and 2, respectively, for paraffin-embedded sections;
- Block non-specific sites in PBS (137 mM NaCl, 5.4 mM KCl, 1.28 mM NaH2PO4, 7 mM Na2HPO4; pH 7.4) with 3% (v/v) normal horse serum (PBS-NHS).
2.2.3. Immunofluorescence
Two-Step Method
- Incubate overnight at room temperature (RT) in primary antibody diluted in PBS-NHS;
- For secondary antibody in Alexa Fluor® PBS-NHS conjugate for 30 min;
- Nuclear counterstain using 500 ng/mL of 4′,6-diamidino-2-phenylindole (DAPI) in PBS;
- Mount using fluorescence-preserving medium (Dako).
Three-Step Method
- 5.
- Primary antibody in PBS-NHS overnight incubation at RT;
- 6.
- Biotinylated secondary antibody in PBS-NHS, incubation for 30 min;
- 7.
- Streptavidin-conjugated fluorochrome incubation for 1 h;
- 8.
- Counterstain with DAPI and mount as above.
2.2.4. Key Points
2.2.5. Disadvantages
- (a)
- The relatively high cost of phosphatase inhibitors;
- (b)
- Despite phosphorylated protein stabilization, this does not lead to antibody signal enhancement.
2.3. Using Organic Solvents and BS3 to Reduce Background
2.3.1. Drawbacks to Face
2.3.2. Organic Solvent Treatment and Crosslinking
- Remove as much culture medium as possible and transfer the coverslip immediately (from 37 to −20 °C as quickly as possible, >5 s) into 25 mL of cold organic solvent (acetone or methanol) at −20 °C and leave for 5 min;
- Remove a coverslip from the organic solvent (acetone or methanol) using forceps and dry vertically in a culture hood until the solvent has completely evaporated (results are better if the solvent is dried quickly);
- Previously, prepare diluted bis(sulfosuccinimidyl)suberate (BS3) by adding 10 µL of 10 mM BS3 to 990 µL PBS, add to PBS 0.1% n-octyl-β-D-glucopyranoside (also known as octyl glucoside [OG]), a nonionic dialyzable detergent that reduces background signal. Incubate samples for 30 min at RT in diluted BS3 crosslinker in a humidified chamber. If the BS3 stain is poor, gentle rehydrate tissue in PBS;
- Remove BS3 carefully, wash it three times with PBS, and then gently wash the buffer;
- Incubate with ethylenediamine (100 mM) for 15 min at RT to quench unreacted BS3;
- Centrifuge blocking buffer (to 10 mL PBS add 0.1 g non-fat powdered milk, 222 μL 45% cold water fish skin gelatin, and 0.1 mL normal horse serum) for 15 min at 2000 g. Transfer supernatant to a new tube and use this blocking buffer from now on.
2.3.3. Antibody Incubations and Mounting
- Use the centrifuged blocking buffer to block non-specific binding sites. Incubate for 1 h at RT;
- Carefully aspirate or blot the blocking buffer. Incubate with primary antibody solution (blocking buffer plus antibody) for 30–60 min at RT;
- Wash gently with blocking buffer eight times;
- Incubate with secondary antibody solution for 30 min at RT;
- Wash with blocking buffer 10 times;
- Mount samples (for homemade mounting medium 90 mL glycerol, add 10 mL of 10X PBS, pH 9 with 0.5 M Na2CO3) and seal the edges with clear nail polish.
2.3.4. Key Points
2.3.5. Disadvantages
- (a)
- Fast dehydration with organic solvents could produce abrupt osmolarity changes and alterations of surface tension.
2.4. Electro-Immunofluorescence
2.4.1. Drawbacks to Face
2.4.2. Methods
- Load IgG conjugates (around 150 kDa) and reagents onto a 1% agarose gel (Figure 4A) in Tris-glycine buffer (25 mM Tris and 250 mM glycine/HCl) [TGB]), pH 7.4;
- Electrophorese at 10 mA for 4–6 h;
- Observation using a UV stereoscopic microscope or gel-documentation system equipped with a fluorescent detector. Fluorescent reagents that migrate towards the positive pole are negatively charged, and vice versa.
2.4.3. Tissue Preparation
- Dissect tissue, fix, and post-fix by immersing in 0.1 M phosphate buffer containing 4% paraformaldehyde and 0.2% glutaraldehyde (this mix of fixatives aims to reduce loss of cytoplasmatic proteins during electrophoresis), pH 7.4, at 4 °C overnight;
- Wash tissues with TGB;
- Incubate with 0.1% Triton X-100 in TGB for 1 h;
- Preparation of column for samples. We recommended hinged plastic containers (Electron Microscopy Sciences #72617-09 or #72617-11) (Figure 4B), or as an alternative, an Eppendorf microtube according to the dimensions of the tissue. The base of the microtube is removed to permit free exchange of TGB within the tube. The microtube containing the sample can be fixed inside an electrophoresis chamber adapting a plastic strip from an Eppendorf tube rack.
2.4.4. Immunoelectrophoresis (Direct Immunofluorescence Method)
- Layer 1: Use the bottom of vial to make layer 1 (if your tissue is polarized place the layer of interest facing up (Figure 4C)), prepare 1% agarose, and 0.05% Triton X-100 in TGB (heat and mix until 50–60 °C, and pour carefully into the vial containing the sample). Wait for polymerization;
- Layer 2: Place an aliquot of 100 μL of 0.1–1 μg (1–10 μg per mL/sample) of primary conjugated antibody in 0.5% agarose (heat and place as point 1) on top of the specimen;
- Layer 3: Seal antibody layer with 100 μL of TGB with 2% agarose (heat and place as point 1);
- Cut the base of the vial or microtube to permit free diffusion of buffer and currents into the column;
- In summary, the column should have three layers (Figure 4D);
- Place the column in the appropriate direction according to the net charge of the staining reagents or conjugated primary antibodies;
- Fill the electrophoresis chamber with 1X TGB;
- Electrophorese for 10–24 h at 4–10 mA;
- Extract tissues, mount the sample, and observe under a laser scanning microscope.
2.4.5. Key Points
2.4.6. Disadvantages
- (a)
- Antibodies will migrate only if they have a different isoelectric point than the TGB used in electrophoresis;
- (b)
- This technique has not been tested for indirect immunolabeling or double and triple immunostaining. If you are looking to apply the two latter methods, the authors suggest the use of antibodies with identical isoelectric points and high binding affinity.
2.5. Antibody Signal Enhancer
2.5.1. Drawbacks to Face
2.5.2. Tissue Preparation
- Transcardial perfusion of mice (3 min) by gravity using 155 mM NaCl, 1 mM MgCl2, 3 mM KCl, 3 mM NaH2PO4, 5 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) pH 7.4, and 1 mM heparin sodium salt dissolved in perfusion solution, added immediately before perfusion into the perfusion system, and if possible, proximal to the animal;
- Transcardial perfusion with fixative solution (30 mM HEPES, 100 mM NaCl, 4% paraformaldehyde, and 0.5% glutaraldehyde, pH 7.4) for 30 min;
- Impregnate for 20 min (do not move the animal after perfusion);
- Remove tissue and place in fresh fixation solution every 12 h for 2 days at 4 °C;
- On the third day, perform final post-fixation in a fresh fixative solution for 2 h at RT.
2.5.3. Immunohistofluorescence
- Cryoprotect using sucrose gradients; 10% overnight, 20%, and 30% 24 h each, or until tissue precipitates to the bottom of the vial at 4 °C;
- Embed in Tissue-Tek OCT compound (Sakura® Finetek) and store at −20 °C for immediate use or at −80 °C for months;
- Obtain 30 μm sections using a cryostat;
- Wash in PBS with 0.5% Tween 20 twice for 3 min;
- Block non-specific sites for 30 min using ASE blocking solution (2% donkey serum, 50 mM glycine, 0.05% Tween 20, 0.1% Triton X-100, and 0.01% bovine serum albumin [BSA] diluted in PBS);
- Incubate in primary antibody diluted in ASE incubation solution (10 mM glycine, 0.05% Tween 20, 0.1% Triton X-100, and 0.1% H2O2 in PBS) overnight at 4 °C;
- Rinse in PBS with 0.5% Tween 20;
- Incubate with secondary antibody diluted in PBS plus 0.1% Tween 20 for 12 h at 4 °C;
- Counterstain nuclei with Hoechst 33342 (Sigma-Aldrich) and mount with DAKO fluorescence mounting medium (DAKO).
2.5.4. Immunocytofluorescence
- Wash cells with PBS;
- Place in methanol at −20 °C for 10 min for fixation and permeabilization;
- Rehydrate at RT with PBS during 10 min;
- Block non-specific sites using ASE blocking solution (see immunohistofluorescence);
- Primary antibody incubation for 1 h at RT in ASE incubation solution (see immunohistofluorescence);
- Rinse thrice with PBS;
- Incubate in Secondary antibody diluted in 0.1% Tween 20 in PBS for 30 min at RT;
- Wash thrice with PBS and twice with deionized water;
- Mount using VectaShield mounting medium.
2.5.5. Key Points
2.5.6. Disadvantages
- (a)
- Take care to not increase more than 0.6% H2O2 [44] when using with fluorescence-conjugated antibodies or reagents, hydrogen peroxidase can quench or bleach the fluorescence;
- (b)
- ASE must always be prepared as a fresh solution.
2.6. Optimized Immunostaining Protocol for Green Fluorescent Protein
2.6.1. Drawbacks to Face
2.6.2. Protocol Applying Transcardial Fixation
- Perfuse with PBS 50 mL (rats) or 20 mL (mice) using a peristaltic pump at a rate of 200 mL/h (rat) or 75–100 mL/h (mouse);
- Perfuse with 150 mL or 30 mL of 4% paraformaldehyde (Sigma Aldrich) for rats and mice, respectively;
- Rinse in PBS and cryoprotect using a sucrose gradient (5% sucrose, 15 min; 15% sucrose, 30 min; sucrose 30% overnight at 4 °C);
- Cryo-embed in OCT (Kaltek) and fast freeze in liquid nitrogen;
- Obtain 10 μm-thick sections using a cryostat;
- Microwave irradiation: Fill Coplin jars with 200 mL of 1 mM ethylenediaminetetraacetic acid (EDTA) pH 8.0, and incubate glass slides. Note: always use the same quantity of coverslips for each Coplin jar. Use an equal volume of buffer and coverslips—use empty coverslips if necessary. Use identical jars and place them in the same position in the microwave oven;
- For frozen sections, perform three consecutive heat–cool cycles for 5 min at 240 W, then cool slowly to 37 °C. The bathing buffer should be between 65 and 68 °C at the end of the heating cycle;
- Permeabilize and incubate with primary antibody using PBS with 1% BSA and 1% Triton X-100 for 12 h at 4 °C and then 2 h at 37 °C;
- Incubate with secondary antibody at 37 °C for 2 h using the antibody incubation buffer from the previous step.
2.6.3. Protocol for Long-Term Fixation by Immersion
- Fix samples with formalin (interval 3 to 6 weeks), and then embed in paraffin (Perintelsint Rvg/2 Kaltek srl);
- Obtain microtome sections (3 µm thick) on glass coverslips (Superfrost plus);
- Deparaffinize cut sections;
- Perform antigen retrieval by immersing the sections in 1 mM EDTA pH 8.0 and irradiating at 750 W using a microwave oven. Boil in a bathing buffer for 45 s and then cool slowly to 45 °C. Repeat the cycle eight times;
- Permeabilize sections in PBS supplemented with 1% BSA and 10% Triton X-100 for 2 h at 37 °C;
- Perform extended permeabilization overnight at 4 °C during primary antibody incubation (see Step 7 of transcardial perfusion);
- Perform secondary antibody incubation diluted in the same buffer of primary antibody.
2.6.4. Key Points
2.6.5. Disadvantages
- (a)
- For optimal parameter control we recommend a specialized laboratory microwave;
- (b)
- High concentrations of Triton X-100 induce membrane damage and autofluorescence [37] and;
- (c)
- excessive paraformaldehyde fixation increases free aldehydes and crosslinking.
2.7. The Super Glue Method: An Improved Technique for Structural Preservation of Retina and Immunofluorescence
2.7.1. Drawbacks to Face
2.7.2. Super Glue Method
- Euthanize animals and collect eyeballs;
- Rinse in PBS pH 7.4;
- Mark eyeballs with a sharpie pen (blue color is recommended by the authors);
- Place eyeballs into a Petri dish, eyecup facing up;
- Remove excess PBS with a Kimwipe®;
- Place super glue (also known as Krazy Glue or Glue 502) into an Eppendorf microtube;
- Dip a 10 μL tip coupled to a pipette into a microtube with super glue, remove immediately;
- Contact the central cornea with the tip and wait for 1 min;
- Dip the sclera attached to the tip pipette briefly into an Eppendorf microtube containing super glue, remove quickly;
- Immerse eyeball immediately in PBS for 1–2 s, allowing the super glue to harden;
- Remove excess PBS with a Kimwipe®;
- Use a syringe needle to poke a hole in the edge of the cornea (it also eliminates pressure and osmotic difference);
- Carefully remove attached tip-cornea and lens;
- Submerge eyecup in paraformaldehyde at RT for 30 min;
- Transfer to 30% sucrose in PBS at RT 30 min;
- Embed in OCT, preserving sagittal plane of the eyecup parallel to the bottom, ultracold at −80 °C;
- Obtain 12 μm sections using a cryostat.
2.7.3. Immunofluorescence Method
- Permeabilize with 5% donkey serum in PBS plus 0.2% Triton X-100;
- Incubate primary antibody diluted in donkey serum, Triton X-100 in PBS at 4 °C overnight;
- Secondary antibody diluted in the same solution RT for 1 h;
- Finally, apply mounting medium anti-fade (Sigma).
2.7.4. Key Points
2.7.5. Disadvantages:
- (a)
- You need to look for alternatives to replace super glue if it is not available in your country. Alternatively, you can test glues that harden immediately with water contact;
- (b)
- Super glue is very sticky and can be spread. Attaching a pipet tip to an eyeball requires practice to avoid attaching other things near to the experiment area.
2.8. Optimized Fixation Using Ultracold Methanol to Improve Immunofluorescence
2.8.1. Drawbacks to Face
2.8.2. Ultracold Methanol Fixation for Immunocytofluorescence
- Wash cells in fresh growth medium or Sorensen’s buffer (15 mM KH2PO4, 2 mM Na2HPO4, pH 6.0);
- Remove excess liquid (but do not over-dry to avoid cell surface tension);
- Using tweezers previously submerged in silicone grease take sapphire coverslips with the cell layer facing upwards and wait for a few seconds;
- Tilt the coverslip with cells at approximately 15° and plunge in ultracold methanol (−85 °C) for a few seconds. Quickly transfer it to another container with ultracold methanol;
- Unfreeze and monitor the coverslip for 30–60 min until its temperature reaches −45 to −40 °C, for plasma membrane or extracellular proteins; use −35 °C for cytoplasmic proteins;
- Plunge the coverslip vertically into PBS at RT;
- Rapidly warm the coverslips and remove excess methanol with gentle movements through the air–buffer interface. Do not move laterally, as this will detach the cell layer. See Figure 8A,B for Steps 4–7;
- Use cells for immunocytofluorescence or store at −20 °C.
2.8.3. Immunofluorescence Protocol
- Incubate coverslips for 30 min in blocking buffer (PBS, 0.2% gelatin and 0.1% Triton X-100);
- Incubate with primary antibody diluted in blocking buffer for 60 min;
- Wash twice for 5 min with PBS;
- Incubate in secondary antibody for 60 min at RT;
- Wash twice for 5 min with PBS;
- Counterstain nuclei with DAPI;
- Plunge in water, dry, and mount using Prolong antifade (Molecular Probes), or alternatively use Fluoromount G (Southern Biotechnology Associates);
- The samples can be stored protected from light for months at 4 °C.
2.8.4. Key Points
2.8.5. Disadvantages
- (a)
- Requires specialized coverslips (made of sapphire);
- (b)
- Rapid-freeze fixation induces the formation of ice crystals, producing cell injury.
2.9. A Simple Immunofluorescence Method to Locate Proteins into the Nucleolus
2.9.1. Drawbacks to Face
2.9.2. Fixation
- Grow cells in monolayers;
- Fix cells in cold (−20 °C) methanol, 10 min.
2.9.3. Immunofluorescence
- Membrane permeabilization with 0.5% Triton X-100;
- Proteinase treatment, 5 min: 50 mg/mL of Proteinase K (Sigma-Aldrich) in PBS at 37 °C. Stop reaction by transferring samples into ice-cold PBS;
- Wash in PBS;
- Blocking step in 1% BSA diluted in PBS, 30 min;
- Primary antibody incubation 60 min at 25 °C;
- Rinse with 0.1% BSA, 0.5% Tween 20 in PBS;
- Secondary antibody incubation 45 min at 25 °C;
- Wash and mount onto slides applying anti-bleaching agent DABCO (Sigma-Aldrich).
2.9.4. Key Points
2.9.5. Disadvantages
- (a)
- Methanol is more effective in locating proteins inside nucleoli, however, this fixative method modifies cellular morphology;
- (b)
- Proteinase induces protein and antigen damage—authors do not recommend coupling this method with an estimation of proteins by quantification and;
- (c)
- Proteinase treatment is only recommended for high amounts of antigens present in high concentrations within the cell.
2.10. Improve Immunofluorescence Combining Heat and Proteolytic-Induced Epitope Retrieval Methods
2.10.1. Drawbacks to Face
2.10.2. Immunofluorescence Formalin-Fixed Paraffin-Embedded Method
- Deparaffinize formalin-fixed 3 μm sections;
- Antigen retrieval treatment: (a) PIER: 0.1% trypsin (Sigma-Aldrich) 30 min at 37 °C, and then (b) HIER: Tris-EDTA buffer pH 9.0, heat into a domestic microwave oven at 750 W (Panasonic). Apply as follows: 5 min of pre-warming, 10 min of heating, and 20 min of cooling at RT;
- Wash with PBS 10 mM pH 7.4;
- Blocking buffer (0.25% casein (Sigma Aldrich) in PBS);
- Primary antibody incubation diluted in blocking buffer 30 min at RT;
- Wash in PBS;
- Secondary antibody incubation for 30 min at RT.
2.10.3. Key Points
2.10.4. Disadvantages
- (a)
- PIER and HIER could generate tissue morphology or epitope damage. Appropriate optimization of incubation, degrees of heating, times, and concentration of chemical reagents are important factors to consider when you apply it to other fixing and embedding methods, and for each antibody. Consider always performing trypsin digestion before microwave treatment to avoid excessive digestion. Tris-EDTA provided better results than the citrate buffer for HIER.
3. Additional Tips
- Drawback to face (Figure 1): 13. As an alternative to avoid artifacts or undesirable stains given by crosslinking between the animal serum and antibodies of similar species, use a buffer free of animal proteins (e.g., “Blocking Reagent, Animal-Free Blocker (5x)–Vector, SP-5030-250”). This solution can be used for immunohistochemistry and immunofluorescence;
- For the antigen retrieval of floating and slide-mounted sections, place floating samples in 1.5 mL tubes or cover-mounted samples (slide mounted) in Coplin jars with a citrate buffer pH 6.0 at 80 °C in a beaker or container. Place in a water bath for 20 min. Temperatures of 80 °C and citrate buffer break cross-bridges generated during fixation;
- Elimination of fluorescent precipitates from fluorochrome-conjugated antibodies: Sometimes old antibodies, or those that are subjected to continuous temperature changes, form precipitates which increase the background signal. Briefly, vortex fluorescent-conjugated antibodies at medium or low velocity for a few seconds, then pulse spin (≈2 s) in a microcentrifuge. Do not invert the tube. Pipette the antibody slightly below the surface of the solution, avoiding the walls of the tube. The precipitates will gather at the bottom of the tube, although the best way to avoid fluorescence precipitates is to aliquot the antibodies when they are totally new;
- Re-dye nuclei counterstaining after photobleaching.
- Protect from light and immerse slices in 96% ethanol;
- Hold slices with fine forceps and carefully remove with a pipette tip the nail varnish of the interface among coverslip and slices;
- Immerse in fresh ethanol 96% until all nail varnish is removed with the tip, or by using a fine instrument if needed;
- Wash three times for 5 min each in PBS;
- Place slices in ethanol 96% 2 min;
- Wash in absolute ethanol for 3 min (twice). Avoid over-drying;
- Perform nuclei counterstain one more time;
- Plunge slices in distilled water or PBS;
- Mount and seal with nail varnish.
- Technique for improving penetration of OCT for frozen sections: Immerse tissues in a 1:1 ratio of: OCT and PBS-0.05%-Tween20-0.1%-Triton X-100-sucrose (consider final concentration of sucrose must be 30% of the total volume). Shake at medium velocity on an orbital shaker, then incubate in whole OCT on a shaker for less than 1 h, and finally rapid freeze;
- Clean slides and coverslips:
- Submerge in 70% ethanol supplemented with 0.01% Tween 20;
- Scrub with a fine and clean brush;
- Immerse in ethanol 96% for a few minutes;
- Move to fresh ethanol 96% again;
- Wiggle each briefly;
- Dry carefully with Kimwipes® (Kimtech), or alternatively use a microwave oven for a few seconds.
- Drawbacks to face (Figure 1): 17. Intense glare gave by counterstaining. Reduce emission spectra and/or pinhole diameter into your confocal parameters to avoid over emission;
- Early membranes permeabilization. During transcardially perfusion: add 0.05% Tween20 and 0.1%-Triton X-100 to wash buffer solution (perfusion or serum replacement). This will increase membrane permeability and, due to the low concentration of detergents, will avoid autofluorescence and membrane damage;
- Drawbacks to face (Figure 1): 3. Transcardial perfusion is a critical step for immunostaining, to minimize hypoxia level and maintain tissue oxygenation during this procedure, apply oxygenated buffer at 37 °C in the washing step (replacement of blood), and it can prevent tissular ischemia;
- The success of immunofluorescence implies a good selection of antibodies, as well as their specific application [57]. For this reason, we provide a final recommendation of two alternative websites for improving your immunostaining, suggested reagents, recipes, equipment, and a protocol database:
- IHC World—Life Sciences Products and Services: As the name implies, it is a full alternative for technical advice for basic concepts, methods, techniques, and protocols; additional sources, such as a forum, blog, distributors, academic or technical jobs, histology issues, and a microscopy section are also included. This webpage displays many sections, and in turn each section displays multiple options that are very useful for beginners and experts in immunolabeling (http://www.ihcworld.com, accessed on 23 November 2021);
- Biocompare, The Buyer’s Guide for Life Scientist: There is a useful database to find antibodies, and this web page includes: antibody suppliers, host species, applications, reactivity, quantity of antibodies, and as very important factors, the number of citations and price are included as well (https://www.biocompare.com//Search-Antibodies/ accessed on 23 November 2021).
4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASE | Antibody signal enhancer |
| BS3 | Bis(sulfosuccinimidyl)suberate |
| BSA | Bovine serum albumin |
| DAPI | 4′,6-diamidino-2-phenylindole |
| EDTA | Ethylenediamine-tetra-acetic acid |
| GFP | Green fluorescent protein |
| HEPES | (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) |
| HIER | Heat-induced epitope retrieval |
| IER | Induced epitope retrieval |
| MAPK | Mitogen-activated protein kinases |
| PBS | Phosphate-buffered saline |
| PBS-NHS | PBS-normal horse serum |
| PIER | Proteolytic-induced epitope retrieval |
| RT | Room temperature |
| TGB | Tris-glycine buffer |
References
- Hsu, S.M.; Raine, L.; Fanger, H. The use of antiavidin antibody and avidin-biotin-peroxidase complex in immunoperoxidase technics. Am. J. Clin. Pathol. 1981, 75, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Flores-Maldonado, C.; Albino-Sánchez, M.E.; Rodríguez-Callejas, J.D.; Estrada-Mondragon, A.; León-Galicia, I.; Maqueda-Alfaro, R.; Perez-Cruz, C.; Fuchs, E.; García-Carrancá, A.; Contreras, R.G.; et al. A Low Cost Antibody Signal Enhancer Improves Immunolabeling in Cell Culture, Primate Brain and Human Cancer Biopsy. Neuroscience 2020, 439, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Kalebi, A.Y.; Dada, M.A. Application of immunohistochemistry in clinical practice: A review. East Afr. Med. J. 2007, 84, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Childs, G.V. History of Immunohistochemistry. Pathobiol. Hum. Dis. A Dyn. Encycl. Dis. Mech. 2014, 3775–3796. [Google Scholar] [CrossRef]
- Sternberger, L.A.; Hardy, P.H.; Cuculis, J.J.; Meyer, H.G. The unlabeled antibody enzyme method of immunohistochemistry: Preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J. Histochem. Cytochem. 1970, 18, 315–333. [Google Scholar] [CrossRef]
- Schnell, S.A.; Staines, W.A.; Wessendorf, M.W. Reduction of lipofuscin-like autofluorescence in fluorescently labeled tissue. J. Histochem. Cytochem. 1999, 47, 719–730. [Google Scholar] [CrossRef] [Green Version]
- Van Epps, H.L. Michael heidelberger and the demystification of antibodies. J. Exp. Med. 2006, 203, 5. [Google Scholar] [CrossRef] [Green Version]
- Sternberger, L.; Cuculis, J. Method for enzymatic intensification of the immunocytochemical reaction without the use of labeled antibodies. J. Histochem. Cytochem. 1969, 17, 190. [Google Scholar]
- Gosselin, E.J.; Dennett, J.C.; Sorenson, G.D.; Pettengill, O.S.; Cate, C.C. Immunocytochemical staining of cytocentrifuge prepared cultured cells: Nonspecific staining and its elimination. Histochem. J. 1985, 17, 847–858. [Google Scholar] [CrossRef]
- Goldberg, M.W. Chapter 7 Immunolabeling for Scanning Electron Microscopy (SEM) and Field Emission SEM. Methods Cell Biol. 2008, 88, 109–130. [Google Scholar] [CrossRef]
- Page Faulk, W.; Malcolm Taylor, G. Communication to the editors. An immunocolloid method for the electron microscope. Immunochemistry 1971, 8, 1081–1083. [Google Scholar] [CrossRef]
- Roth, J. The silver anniversary of gold: 25 years of the colloidal gold marker system for immunocytochemistry and histochemistry. Histochem. Cell Biol. 1996, 106, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Im, K.; Mareninov, S.; Diaz, M.F.P.; Yong, W.H. An Introduction to Performing Immunofluorescence Staining. Methods Mol. Biol. 2019, 1897, 299–311. [Google Scholar] [CrossRef]
- Mölne, J.; Breimer, M.E.; Svalander, C.T. Immunoperoxidase versus immunofluorescence in the assessment of human renal biopsies. Am. J. Kidney Dis. 2005, 45, 674–683. [Google Scholar] [CrossRef]
- Rosas-Arellano, A.; Villalobos-González, J.B.; Palma-Tirado, L.; Beltrán, F.A.; Cárabez-Trejo, A.; Missirlis, F.; Castro, M.A. A simple solution for antibody signal enhancement in immunofluorescence and triple immunogold assays. Histochem. Cell Biol. 2016, 146, 421–430. [Google Scholar] [CrossRef]
- Grube, D.; Kusumoto, Y. Serial semithin sections in immunohistochemistry: Techniques and applications. Arch. Histol. Jpn. 1986, 49, 391–410. [Google Scholar] [CrossRef] [Green Version]
- Grube, D. Constants and variables in immunohistochemistry. Arch. Histol. Cytol. 2004, 67, 115–134. [Google Scholar] [CrossRef] [Green Version]
- Berghorn, K.A.; Bonnett, J.H.; Hoffman, G.E. cFos immunoreactivity is enhanced with biotin amplification. J. Histochem. Cytochem. 1994, 42, 1635–1642. [Google Scholar] [CrossRef] [Green Version]
- Reid, F.L.; Hall, N.H.; Smith, J.S.; Baer, G.M. Increased immunofluorescent staining of rabies-infected, formalin-fixed brain tissue after pepsin and trypsin digestion. J. Clin. Microbiol. 1983, 18, 968–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, S.R.; Key, M.E.; Kalra, K.L. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: An enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J. Histochem. Cytochem. 1991, 39, 741–748. [Google Scholar] [CrossRef] [Green Version]
- Adams, J.C. Biotin amplification of biotin and horseradish peroxidase signals in histochemical stains. J. Histochem. Cytochem. 1992, 40, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Vidunas, A.J.; Beniash, E. Optimizing Immunostaining of Enamel Matrix: Application of Sudan Black B and Minimization of False Positives from Normal Sera and IgGs. Front. Physiol. 2017, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Taatjes, D.J.; Roth, J. In Focus in HCB. Histochem. Cell Biol. 2016, 146, 363–365. [Google Scholar] [CrossRef] [Green Version]
- Zaglia, T.; Di Bona, A.; Chioato, T.; Basso, C.; Ausoni, S.; Mongillo, M. Optimized protocol for immunostaining of experimental GFP-expressing and human hearts. Histochem. Cell Biol. 2016, 146, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Kao, W.W.Y. A novel protocol of whole mount electro-immunofluorescence staining. Mol. Vis. 2009, 15, 505. [Google Scholar]
- Yabuki, A.; Sawa, M.; Kohyama, M.; Hamamoto, T.; Yamato, O. Paraffin immunofluorescence for detection of immune complexes in renal biopsies: An efficient salvage technique for diagnosis of glomerulonephritis in dogs. BMC Vet. Res. 2017, 13, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, G.E.; Murphy, K.J.; Sita, L.V. The Importance of Titrating Antibodies for Immunocytochemical Methods. Curr. Protoc. Neurosci. 2016, 76, 2.12.1–2.12.37. [Google Scholar] [CrossRef] [Green Version]
- Hagedorn, M.; Neuhaus, E.M.; Soldati, T. Optimized fixation and immunofluorescence staining methods for Dictyostelium cells. Methods Mol. Biol. 2006, 346, 327–338. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, D.; Hammond, A.T.; Glick, B.S. High-Quality Immunofluorescence of Cultured Cells. Methods Mol. Biol. 2010, 619, 403–410. [Google Scholar] [CrossRef] [Green Version]
- Mammone, T.; Chidlow, G.; Casson, R.J.; Wood, J.P.M. Improved immunohistochemical detection of phosphorylated mitogen-activated protein kinases in the injured rat optic nerve head. Histochem. Cell Biol. 2019, 151, 435–456. [Google Scholar] [CrossRef]
- Svistunova, D.M.; Musinova, Y.R.; Polyakov, V.Y.; Sheval, E.V. A Simple Method for the Immunocytochemical Detection of Proteins Inside Nuclear Structures That Are Inaccessible to Specific Antibodies. J. Histochem. Cytochem. 2012, 60, 152. [Google Scholar] [CrossRef]
- Yang, J.; Zou, T.; Yang, F.; Zhang, Z.; Sun, C.; Yang, Z.; Zhang, H. A quick protocol for the preparation of mouse retinal cryosections for immunohistochemistry. Open Biol. 2021, 11, 210076. [Google Scholar] [CrossRef]
- Battifora, H. Assessment of antigen damage in immunohistochemistry. The vimentin internal control. Am. J. Clin. Pathol. 1991, 96, 669–671. [Google Scholar] [CrossRef]
- Ghrebi, S.S.; Owen, G.R.; Brunette, D.M. Triton X-100 Pretreatment of LR-white Thin Sections Improves Immunofluorescence Specificity and Intensity. Microsc. Res. Tech. 2007, 70, 555–562. [Google Scholar] [CrossRef]
- Migheli, A.; Attanasio, A.; Schiffer, D. LR Gold embedding of nervous tissue for immunoelectron microscopy studies. Histochemistry 1992, 97, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Mattei, B.; Lira, R.B.; Perez, K.R.; Riske, K.A. Membrane permeabilization induced by Triton X-100: The role of membrane phase state and edge tension. Chem. Phys. Lipids 2017, 202, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Suah, F.B.M.; Ahmad, M.; Mehamod, F.S. Effect of non-ionic surfactants to the Al(III)-morin complex and its application in determination of Al(III) ions: A preliminary study. Malays. J. Anal. Sci. 2017, 21, 793–800. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, W.Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. Res. 2015, 35, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Blazek, M.; Santisteban, T.S.; Zengerle, R.; Meier, M. Analysis of fast protein phosphorylation kinetics in single cells on a microfluidic chip. Lab Chip 2015, 15, 726–734. [Google Scholar] [CrossRef] [Green Version]
- Monast, C.S.; Furcht, C.M.; Lazzara, M.J. Computational analysis of the regulation of EGFR by protein tyrosine phosphatases. Biophys. J. 2012, 102, 2012–2021. [Google Scholar] [CrossRef] [Green Version]
- Start, R.D.; Layton, C.M.; Cross, S.S.; Smith, J.H.F. Reassessment of the rate of fixative diffusion. J. Clin. Pathol. 1992, 45, 1120. [Google Scholar] [CrossRef] [Green Version]
- Ezaki, T. Antigen retrieval: Its significance and drawbacks in immunohistochemistry. Kaibogaku Zasshi 1996, 71, 615–628. [Google Scholar]
- Piekut, D.T.; Casey, S.M. Penetration of immunoreagents in Vibratome-sectioned brain: A light and electron microscopic study. J. Histochem. Cytochem. 1983, 31, 669–674. [Google Scholar] [CrossRef] [Green Version]
- Govender, D.; Davids, L.M.; Kidson, S.H. Immunofluorescent Identification of Melanocytes in Murine Hair Follicles. J. Mol. Histol. 2006, 37, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, O. Discovery of green fluorescent protein. Methods Biochem. Anal. 2006, 47, 1–13. [Google Scholar] [CrossRef]
- Mavrakis, M.; Pourquié, O.; Lecuit, T. Lighting up developmental mechanisms: How fluorescence imaging heralded a new era. Development 2010, 137, 373–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, P.M.; Albrecht, D.; Culley, S.; Jacobs, C.; Marsh, M.; Mercer, J.; Henriques, R. Fix your membrane receptor imaging: Actin cytoskeleton and CD4 membrane organization disruption by chemical fixation. Front. Immunol. 2019, 10, 675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.W.; Zhou, L.; Lam, H.M.; Langowski, J. Paraformaldehyde Fixation May Lead to Misinterpretation of the Subcellular Localization of Plant High Mobility Group Box Proteins. PLoS ONE 2015, 10, e135033. [Google Scholar] [CrossRef]
- Claybon, A.; Bishop, A.J.R. Dissection of a mouse eye for a whole mount of the retinal pigment epithelium. J. Vis. Exp. 2011, 48, 2563. [Google Scholar] [CrossRef] [Green Version]
- Masland, R.H. The fundamental plan of the retina. Nat. Neurosci. 2001, 4, 877–886. [Google Scholar] [CrossRef]
- Chiu, K.; Chan, T.F.; Wu, A.; Leung, I.Y.P.; So, K.F.; Chang, R.C.C. Neurodegeneration of the retina in mouse models of Alzheimer’s disease: What can we learn from the retina? Age 2012, 34, 633. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Lin, Y.; Yang, F.; Zou, T.; Yang, J.; Zhang, H. An improved method for preparation of mouse retinal cryosections. Eur. J. Histochem. 2020, 64, 1–5. [Google Scholar] [CrossRef]
- Neuhaus, E.M.; Horstmann, H.; Almers, W.; Maniak, M.; Soldati, T. Ethane-Freezing/Methanol-Fixation of Cell Monolayers: A Procedure for Improved Preservation of Structure and Antigenicity for Light and Electron Microscopies. J. Struct. Biol. 1998, 121, 326–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pegg, D.E. The relevance of ice crystal formation for the cryopreservation of tissues and organs. Cryobiology 2010, 60, S36–S44. [Google Scholar] [CrossRef] [PubMed]
- Tiku, V.; Antebi, A. Nucleolar Function in Lifespan Regulation. Trends Cell Biol. 2018, 28, 662–672. [Google Scholar] [CrossRef]
- Thavarajah, R.; Mudimbaimannar, V.K.; Elizabeth, J.; Rao, U.K.; Ranganathan, K. Chemical and physical basics of routine formaldehyde fixation. J. Oral Maxillofac. Pathol. 2012, 16, 400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acharya, P.; Quinlan, A.; Neumeister, V. The ABCs of finding a good antibody: How to find a good antibody, validate it, and publish meaningful data. F1000Research 2017, 6, 851. [Google Scholar] [CrossRef]
- Saper, C.B. An open letter to our readers on the use of antibodies. J. Comp. Neurol. 2005, 493, 477–478. [Google Scholar] [CrossRef]
- Echeazarra, L.; García del Caño, G.; Barrondo, S.; González-Burguera, I.; Saumell-Esnaola, M.; Aretxabala, X.; López de Jesús, M.; Borrega-Román, L.; Mato, S.; Ledent, C.; et al. Fit-for-purpose based testing and validation of antibodies to amino- and carboxy-terminal domains of cannabinoid receptor 1. Histochem. Cell Biol. 2021, 156, 479–502. [Google Scholar] [CrossRef]
- Bordeaux, J.; Welsh, A.W.; Agarwal, S.; Killiam, E.; Baquero, M.T.; Hanna, J.A.; Anagnostou, V.K.; Rimm, D.L. Antibody validation. Biotechniques 2010, 48, 197–209. [Google Scholar] [CrossRef] [Green Version]
- Uhlen, M.; Bandrowski, A.; Carr, S.; Edwards, A.; Ellenberg, J.; Lundberg, E.; Rimm, D.L.; Rodriguez, H.; Hiltke, T.; Snyder, M.; et al. A proposal for validation of antibodies. Nat. Methods 2016, 13, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Saper, C.B.; Sawchenko, P.E. Magic peptides, magic antibodies: Guidelines for appropriate controls for immunohistochemistry. J. Comp. Neurol. 2003, 465, 161–163. [Google Scholar] [CrossRef] [PubMed]










| Enzymes | Fluorochromes | |
|---|---|---|
| Prefix | Suffix | |
| Immuno | Immunochemistry (also immunoenzyme) | Immunofluorescence |
| Cyto (cell) | Cytochemistry | Cytofluorescence |
| Histo (tissue) | Histochemistry | Histofluorescence |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piña, R.; Santos-Díaz, A.I.; Orta-Salazar, E.; Aguilar-Vazquez, A.R.; Mantellero, C.A.; Acosta-Galeana, I.; Estrada-Mondragon, A.; Prior-Gonzalez, M.; Martinez-Cruz, J.I.; Rosas-Arellano, A. Ten Approaches That Improve Immunostaining: A Review of the Latest Advances for the Optimization of Immunofluorescence. Int. J. Mol. Sci. 2022, 23, 1426. https://doi.org/10.3390/ijms23031426
Piña R, Santos-Díaz AI, Orta-Salazar E, Aguilar-Vazquez AR, Mantellero CA, Acosta-Galeana I, Estrada-Mondragon A, Prior-Gonzalez M, Martinez-Cruz JI, Rosas-Arellano A. Ten Approaches That Improve Immunostaining: A Review of the Latest Advances for the Optimization of Immunofluorescence. International Journal of Molecular Sciences. 2022; 23(3):1426. https://doi.org/10.3390/ijms23031426
Chicago/Turabian StylePiña, Ricardo, Alma I. Santos-Díaz, Erika Orta-Salazar, Azucena Ruth Aguilar-Vazquez, Carola A. Mantellero, Isabel Acosta-Galeana, Argel Estrada-Mondragon, Mara Prior-Gonzalez, Jadir Isai Martinez-Cruz, and Abraham Rosas-Arellano. 2022. "Ten Approaches That Improve Immunostaining: A Review of the Latest Advances for the Optimization of Immunofluorescence" International Journal of Molecular Sciences 23, no. 3: 1426. https://doi.org/10.3390/ijms23031426