Effects of Cardiac Stem Cell on Postinfarction Arrhythmogenic Substrate
Abstract
:1. Introduction
2. Results
2.1. Scar and Left Ventricle Volume Changes
2.2. Electrophysiological Study and Electro-Anatomical Mapping
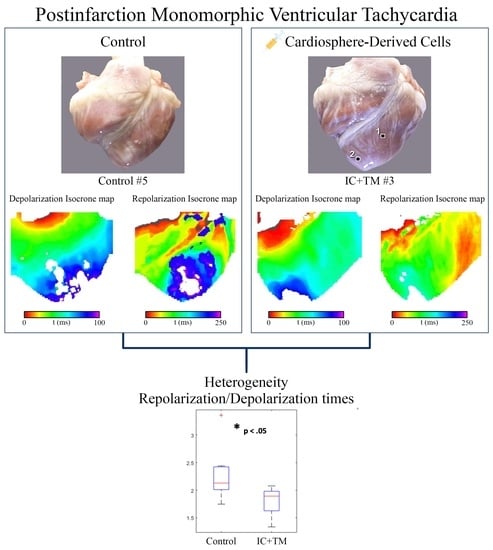
2.3. Optical Mapping
2.3.1. Mapping during Epicardial Pacing
2.3.2. Mapping during VT
2.4. Histological Analysis
2.4.1. Dense Scar
2.4.2. Heterogeneous Tissue
2.4.3. Subendocardium
2.4.4. Epicardial Intra-Scar Channels
3. Discussion
3.1. Structural and Electrophysiological Remodeling in Healed Infarct
- The dense scar replaces myocyte loss at the border zone. In this case, the primary event is myocyte death.
- Dense scar growth is the primary event, thus dense scar surrounds and isolates myocytes, expediting cellular death somehow.
3.2. Effects of CDCs on Structural and Electrophysiological Remodeling in Healed Infarct
3.3. Safety Concerns of Stem Cell Therapy
3.4. Limitations
4. Materials and Methods
4.1. Ventricular Tachycardia Substrate Evaluation Sub-Study
4.2. Cell Isolation and Production
4.2.1. Isolation and Production of Porcine Cardiosphere-Derived Cells
4.2.2. Characterization of Cardiosphere-Derived Cells
4.3. Intracoronary and Transmyocardial Cell Delivery
4.4. Electrophysiological Study and Electro-Anatomical Mapping
4.5. Optical Mapping
4.6. Histological Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Anesthesia and Monitoring Protocols for Infarct Induction, MRI Examination, and Electrophysiological Studies
Appendix A.2. Infarct Induction
Appendix A.3. Magnetic Resonance Imaging
Appendix A.4. Magnetic Resonance-Based Endo-Epicardial Signal Intensity Mapping
Appendix A.5. Optical Mapping
Appendix A.6. Histological Analysis
References
- Solomon, S.D.; Zelenkofske, S.; McMurray, J.J.V.; Finn, P.V.; Velazquez, E.; Ertl, G.; Harsanyi, A.; Rouleau, J.L.; Maggioni, A.; Kober, L.; et al. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N. Engl. J. Med. 2005, 352, 2581–2588. [Google Scholar] [CrossRef] [PubMed]
- Hohnloser, S.H.; Kuck, K.H.; Dorian, P.; Roberts, R.S.; Hampton, J.R.; Hatala, R.; Fain, E.; Gent, M.; Connolly, S.J. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N. Engl. J. Med. 2004, 351, 2481–2488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinbeck, G.; Andresen, D.; Seidl, K.; Brachmann, J.; Hoffmann, E.; Wojciechowski, D.; Kornacewicz-Jach, Z.; Sredniawa, B.; Lupkovics, G.; Hofgärtner, F.; et al. Defibrillator Implantation Early after Myocardial Infarction. N. Engl. J. Med. 2009, 361, 1427–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poole, J.; Johnson, G.; Hellkamp, A.; Anderson, J.; Callans, D.; Raitt, M.; Reddy, R.; Marchlinski, F.; Yee, R.; Guarnieri, T.; et al. Prognostic importance of defibrillator shocks in patients with heart failure. N. Engl. J. Med. 2008, 359, 1009–1017. [Google Scholar] [CrossRef] [Green Version]
- Sasano, T.; McDonald, A.D.; Kikuchi, K.; Donahue, J.K. Molecular Ablation of Ventricular Tachycardia after Myocardial Infarction. Nat. Med. 2006, 12, 1256. [Google Scholar] [CrossRef]
- Greener, I.D.; Sasano, T.; Wan, X.; Igarashi, T.; Strom, M.; Rosenbaum, D.S.; Donahue, J.K. Connexin43 gene transfer reduces ventricular tachycardia susceptibility after myocardial infarction. J. Am. Coll. Cardiol. 2012, 60, 1103–1110. [Google Scholar] [CrossRef] [Green Version]
- Makkar, R.R.; Smith, R.R.; Cheng, K.; Malliaras, K.; Thomson, L.E.J.; Berman, D.; Czer, L.S.C.; Marbán, L.; Mendizabal, A.; Johnston, P.V.; et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): A prospective, randomised phase 1 trial. Lancet 2012, 379, 895–904. [Google Scholar] [CrossRef] [Green Version]
- Makkar, R.R.; Kereiakes, D.J.; Aguirre, F.; Kowalchuk, G.; Chakravarty, T.; Malliaras, K.; Francis, G.S.; Povsic, T.J.; Schatz, R.; Traverse, J.H.; et al. Intracoronary ALLogeneic heart STem cells to Achieve myocardial Regeneration (ALLSTAR): A randomized, placebo-controlled, double-blinded trial. Eur. Heart J. 2020, 41, 3451–3458. [Google Scholar] [CrossRef]
- Afzal, M.R.; Samanta, A.; Shah, Z.I.; Jeevanantham, V.; Abdel-Latif, A.; Zuba-Surma, E.K.; Dawn, B. Adult Bone Marrow Cell Therapy for Ischemic Heart Disease: Evidence and Insights From Randomized Controlled Trials. Circ. Res. 2015, 117, 558–575. [Google Scholar] [CrossRef] [Green Version]
- Jiang, M.; Cabo, C.; Yao, J.A.; Boyden, P.A.; Tseng, G.N. Delayed rectifier K currents have reduced amplitudes and altered kinetics in myocytes from infarcted canine ventricle. Cardiovasc. Res. 2000, 48, 34–43. [Google Scholar] [CrossRef]
- Marfella, R.; Rizzo, M.R.; Siniscalchi, M.; Paolisso, P.; Barbieri, M.; Sardu, C.; Savinelli, A.; Angelico, N.; Del Gaudio, S.; Esposito, N.; et al. Peri-procedural tight glycemic control during early percutaneous coronary intervention up-regulates endothelial progenitor cell level and differentiation during acute ST-elevation myocardial infarction: Effects on myocardial salvage. Int. J. Cardiol. 2013, 168, 3954–3962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramkisoensing, A.A.; De Vries, A.A.F.; Atsma, D.E.; Schalij, M.J.; Pijnappels, D.A. Interaction between myofibroblasts and stem cells in the fibrotic heart: Balancing between deterioration and regeneration. Cardiovasc. Res. 2014, 102, 224–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paolisso, P.; Bergamaschi, L.; Santulli, G.; Gallinoro, E.; Cesaro, A.; Gragnano, F.; Sardu, C.; Mileva, N.; Foà, A.; Armillotta, M.; et al. Infarct size, inflammatory burden, and admission hyperglycemia in diabetic patients with acute myocardial infarction treated with SGLT2-inhibitors: A multicenter international registry. Cardiovasc. Diabetol. 2022, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Miragoli, M.; Gaudesius, G.; Rohr, S. Electrotonic Modulation of Cardiac Impulse Conduction by Myofibroblasts. Circ. Res. 2006, 98, 801–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, S.A.; Burridge, P.W.; Lipke, E.A.; Shamblott, M.; Zambidis, E.T.; Tung, L. Engraftment of human embryonic stem cell derived cardiomyocytes improves conduction in an arrhythmogenic in vitro model. J. Mol. Cell. Cardiol. 2012, 53, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.T.; White, A.J.; Matsushita, S.; Malliaras, K.; Steenbergen, C.; Zhang, Y.; Li, T.S.; Terrovitis, J.; Yee, K.; Simsir, S.; et al. Intramyocardial injection of autologous cardiospheres or cardiosphere-derived cells preserves function and minimizes adverse ventricular remodeling in pigs with heart failure post-myocardial infarction. J. Am. Coll. Cardiol. 2011, 57, 455–465. [Google Scholar] [CrossRef] [Green Version]
- Menasché, P. Stem cell therapy for heart failure: Are arrhythmias a real safety concern? Circulation 2009, 119, 2735–2740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Avilés, F.; Sanz-Ruiz, R.; Bogaert, J.; Plasencia, A.C.; Gilaberte, I.; Belmans, A.; Fernández-Santos, M.E.; Charron, D.; Mulet, M.; Yotti, R.; et al. Safety and Efficacy of Intracoronary Infusion of Allogeneic Human Cardiac Stem Cells in Patients With ST-Segment Elevation Myocardial Infarction and Left Ventricular Dysfunction. Circ. Res. 2018, 123, 579–589. [Google Scholar] [CrossRef]
- Arenal, Á.; Pérez-David, E.; Ávila, P.; Fernández-Portales, J.; Crisóstomo, V.; Báez, C.; Jiménez-Candil, J.; Rubio-Guivernau, J.L.; Ledesma-Carbayo, M.J.; Loughlin, G.; et al. Noninvasive identification of epicardial ventricular tachycardia substrate by magnetic resonance-based signal intensity mapping. Hear. Rhythm 2014, 11, 1456–1464. [Google Scholar] [CrossRef]
- Hummel, J.D.; Strickberger, S.A.; Daoud, E.; Niebauer, M.; Bakr, O.; Man, K.C.; Williamson, B.D.; Morady, F. Results and efficiency of programmed ventricular stimulation with four extrastimuli compared with one, two, and three extrastimuli. Circulation 1994, 90, 2827–2832. [Google Scholar] [CrossRef]
- Bayly, P.V.; KenKnight, B.H.; Rogers, J.M.; Hillsley, R.E.; Ideker, R.E.; Smith, W.M. Estimation of conduction velocity vector fields from epicardial mapping data. IEEE Trans. Biomed. Eng. 1998, 45, 563–571. [Google Scholar] [CrossRef] [PubMed]









| Control (N = 11) | IC+IM Cells (N = 9) | p-Value | |
|---|---|---|---|
| Scar 4W (cc) | 7.4 ± 3.2 | 8.2 ± 3.0 | ns |
| Scar 16W (cc) | 11.0 ± 4.0 | 11.2 ± 4.0 | ns |
| ∆Scar (%) | 80.0 ± 80.0 | 36.0 ± 17.0 | 0.1 |
| Dense Scar 4W (cc) | 3.9 ± 2.0 | 4.1 ± 2.2 | ns |
| Dense Scar 16W (cc) | 6.1 ± 1.7 | 5.5 ± 3.0 | ns |
| ∆Dense Scar (%) | 77.0 ± 70.0 | 31.0 ± 12.0 | 0.02 |
| HT 4W (cc) | 3.5 ± 1.5 | 4.0 ± 1.1 | ns |
| HT 16W (cc) | 5.8 ± 2.7 | 5.6 ± 1.6 | ns |
| ∆HT (%) | 64.0 ± 69.0 | 42.0 ± 31.0 | ns |
| LVEF % (4W) | 44.0 ± 6.0 | 40.0 ± 6.0 | 0.1 |
| LVEF % (16W) | 39.0 ± 5.0 | 40.0 ± 6.0 | 0.9 |
| ∆LVEF (%) | −10 ± 14.0 | 1.0 ± 8.0 | 0.04 |
| Scar 4W (cc) | 7.4 ± 3.2 | 8.2 ± 3.0 | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arenal, Á.; Ríos-Muñoz, G.R.; Carta-Bergaz, A.; Ruiz-Hernández, P.M.; Pérez-David, E.; Crisóstomo, V.; Loughlin, G.; Sanz-Ruiz, R.; Fernández-Portales, J.; Acosta, A.; et al. Effects of Cardiac Stem Cell on Postinfarction Arrhythmogenic Substrate. Int. J. Mol. Sci. 2022, 23, 16211. https://doi.org/10.3390/ijms232416211
Arenal Á, Ríos-Muñoz GR, Carta-Bergaz A, Ruiz-Hernández PM, Pérez-David E, Crisóstomo V, Loughlin G, Sanz-Ruiz R, Fernández-Portales J, Acosta A, et al. Effects of Cardiac Stem Cell on Postinfarction Arrhythmogenic Substrate. International Journal of Molecular Sciences. 2022; 23(24):16211. https://doi.org/10.3390/ijms232416211
Chicago/Turabian StyleArenal, Ángel, Gonzalo R. Ríos-Muñoz, Alejandro Carta-Bergaz, Pablo M. Ruiz-Hernández, Esther Pérez-David, Verónica Crisóstomo, Gerard Loughlin, Ricardo Sanz-Ruiz, Javier Fernández-Portales, Alejandra Acosta, and et al. 2022. "Effects of Cardiac Stem Cell on Postinfarction Arrhythmogenic Substrate" International Journal of Molecular Sciences 23, no. 24: 16211. https://doi.org/10.3390/ijms232416211