A Comprehensive Study of Gemfibrozil Complexation with β-Cyclodextrins in Aqueous Solution Using Different Analytical Techniques
Abstract
:1. Introduction
2. Results and Discussion
2.1. Nuclear Magnetic Resonance (1H-NMR) Spectroscopy
2.2. Fluorescence Spectroscopy
2.3. Solubility Isotherms
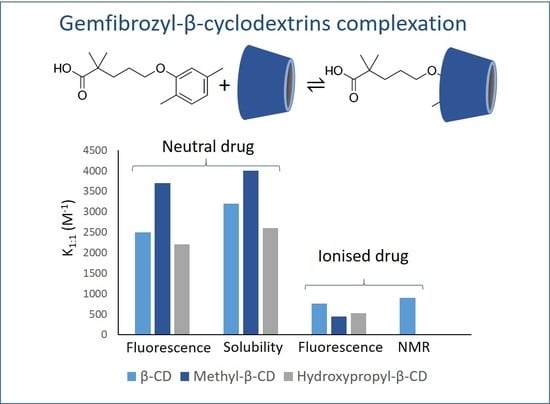
2.4. Remarks on the Suitability of the Different Experimental Techniques Used to Determine the Binding Constants of Cyclodextrins Complexes in Solution
2.5. Solid State Complexation
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. 1H Nuclear Magnetic Resonance (1H-NMR) Spectroscopy
3.2.2. Fluorescence Spectroscopy
3.2.3. Solubility Isotherms
3.2.4. Preparation and Characterization of Solid Dispersions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aiassa, V.; Garnero, C.; Longhi, M.R.; Zoppi, A. Cyclodextrin multicomponent complexes: Pharmaceutical applications. Pharmaceutics 2021, 13, 1099. [Google Scholar] [CrossRef] [PubMed]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gaitano, G.; Isasi, J.; Velaz, I.; Zornoza, A. Drug Carrier Systems Based on Cyclodextrin Supramolecular Assemblies and Polymers: Present and Perspectives. Curr. Pharm. Des. 2016, 23, 411–432. [Google Scholar] [CrossRef] [PubMed]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J.; Osa, T. (Eds.) Cyclodextrins, Vol. 3 of Comprehensive Supramolecular Chemistry, 1st ed.; Elsevier Science: Oxford, UK, 1996. [Google Scholar]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.P.; Wang, J.X.; Chen, G.Z.; Shen, Z.G.; Chen, J.F.; Yun, J. Micronization of gemfibrozil by reactive precipitation process. Int. J. Pharm. 2008, 360, 58–64. [Google Scholar] [CrossRef]
- Fernández, L.; Martínez-Ohárriz, M.C.; Martín, C.; Vélaz, I.; Sánchez, M.; Zornoza, A. Analysis of the complexation of gemfibrozil with γ- and hydroxypropyl-γ-cyclodextrins. J. Pharm. Biomed. Anal. 2008, 47, 943–948. [Google Scholar] [CrossRef]
- Aigner, Z.; Berkesi, O.; Farkas, G.; Szabó-Révész, P. DSC, X-ray and FTIR studies of a gemfibrozil/dimethyl-β-cyclodextrin inclusion complex produced by co-grinding. J. Pharm. Biomed. Anal. 2012, 57, 62–67. [Google Scholar] [CrossRef]
- Fernández, M.S.L.; Martín, C.; Martínez-Ohárriz, M.C.; Zornoza, A.; Vélaz, I.; González-Gaitano, G. Study of the interaction of β-cyclodextrins with gemfibrozil using a spectrofluorimetric method. In Proceedings of the 12th International Cyclodextrin Symposium, Montpellier, France, 16–19 May 2004; pp. 275–278. [Google Scholar]
- Carrier, R.L.; Miller, L.A.; Ahmed, I. The utility of cyclodextrins for enhancing oral bioavailability. J. Control. Release 2007, 123, 78–99. [Google Scholar] [CrossRef]
- Huang, H.; Liu, F.; Jia, B.X.; Xu, K.H.; Chen, Z.Z.; Tang, B. Study on the supramolecular interaction of β-cyclodextrin with gemfibrozil by spectrofluorimetry and its analytical application. Chin. J. Chem. 2007, 25, 337–342. [Google Scholar] [CrossRef]
- Maddens, T.; Vélaz, I.; Machín, R.; Isasi, J.R.; Martín, C.; Martínez-Ohárriz, M.C.; Zornoza, A. Complexation of ebastine with β-cyclodextrin derivatives. J. Incl. Phenom. Macrocycl. Chem. 2011, 70, 415–419. [Google Scholar] [CrossRef]
- Loftsson, T.; Hreinsdóttir, D.; Másson, M. The complexation efficiency. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 545–552. [Google Scholar] [CrossRef]
- Manzoori, J.L.; Amjadi, M. Spectrofluorimetric and micelle-enhanced spectrofluorimetric methods for the determination of gemfibrozil in pharmaceutical preparations. J. Pharm. Biomed. Anal. 2003, 31, 507–513. [Google Scholar] [CrossRef]
- Tang, B.; Jia, B.; Cui, G.; Ding, Y. Study on the supramolecular interaction between β-cyclodextrin and gemfibrozil by flow injection spectrofluorimetry and its analytical application. Anal. Chim. Acta 2004, 516, 221–227. [Google Scholar] [CrossRef]
- Ross, P.D.; Rekharsky, M.V. Thermodynamics of hydrogen bond and hydrophobic interactions in cyclodextrin complexes. Biophys. J. 1996, 71, 2144–2154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of cyclodextrins and drug/cyclodextrin complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anguiano-Igea, S.; Otero-Espinar, F.J.; Vila-Jato, J.L.; Blanco-Méndez, J. Interaction of clofibrate with cyclodextrin in solution: Phase solubility, 1H NMR and molecular modelling studies. Eur. J. Pharm. Sci. 1997, 5, 215–221. [Google Scholar] [CrossRef]
- Ding, X.; Zheng, M.; Lu, J.; Zhu, X. Preparation and evaluation of binary and ternary inclusion complexes of fenofibrate/hydroxypropyl-β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2018, 91, 17–24. [Google Scholar] [CrossRef]
- Lucio, D.; Irache, J.M.; Font, M.; Martínez-Ohárriz, M.C. Nanoaggregation of inclusion complexes of glibenclamide with cyclodextrins. Int. J. Pharm. 2017, 519, 263–271. [Google Scholar] [CrossRef]
- González-Gaitano, G.; Sainz-Rozas, P.R.; Isasi, J.R.; Guerrero-Martínez, A.; Tardajos, G. Site-specific interactions between 2-dibenzofuran carboxylate and β- and ϒ-cyclodextrins determined by intermolecular NOE and molecular modelling. J. Phys. Chem. B 2004, 108, 14154–14162. [Google Scholar] [CrossRef]
- Parker, C.A.; Rees, W.T. Correction of fluorescence spectra and measurement of fluorescence quantum efficiency. Analyst 1960, 85, 587–600. [Google Scholar] [CrossRef]
- Connors, K.A. Binding Constants: The Measurement of Molecular Complex Stability, 1st ed.; John Wiley & Sons: New York, NY, USA, 1987. [Google Scholar]
- Higuchi, K.A.; Connors, T. Advances in Analytical Chemistry and Instrumentation; Wiley-Interscience: Ney York, NY, USA, 1965; Volume 4. [Google Scholar]
- Mura, P. Analytical techniques for characterization of cyclodextrin complexes in aqueous solution: A review. J. Pharm. Biomed. Anal. 2014, 101, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Mura, P. Analytical techniques for characterization of cyclodextrin complexes in the solid state: A review. J. Pharm. Biomed. Anal. 2015, 113, 226–238. [Google Scholar] [CrossRef] [PubMed]





| ΦpH 2.8 | ΦpH 7.0 | |
|---|---|---|
| Gemfibrozil | 0.22 ± 0.02 | 0.23 ± 0.01 |
| Gemfibrozil: β-CD | 0.30 ± 0.02 | 0.36 ± 0.01 |
| Gemfibrozil: Mβ-CD | 0.34 ± 0.03 | 0.36 ± 0.01 |
| Gemfibrozil: HPβ-CD | 0.33 ± 0.03 | 0.36 ± 0.01 |
| pH 2.8 | pH 7.0 | |||
|---|---|---|---|---|
| T (°C) | K1:1 10−2 (M−1) | a | K1:1 10−2 (M−1) | a |
| β-CD | ||||
| 15 | 36 ± 2 | 1.45 ± 0.04 | 9.9 ± 0.2 | 1.42 ± 0.03 |
| 25 | 25 ± 2 | 1.62 ± 0.04 | 7.6 ± 0.3 | 1.58 ± 0.01 |
| 35 | 17 ± 1 | 1.81 ± 0.04 | 6.3 ± 0.2 | 1.85 ± 0.04 |
| 45 | 12 ± 2 | 2.09 ± 0.02 | 5.2 ± 0.2 | 2.30 ± 0.02 |
| Meβ-CD | ||||
| 15 | 42 ± 3 | 1.62 ± 0.03 | 5.1 ± 0.2 | 1.51 ± 0.05 |
| 25 | 37 ± 2 | 1.73 ± 0.02 | 4.4 ± 0.2 | 1.75 ± 0.06 |
| 35 | 33 ± 2 | 1.92 ± 0.03 | 3.8 ± 0.2 | 2.11 ± 0.04 |
| 45 | 28 ± 3 | 2.23 ± 0.03 | 3.4 ± 0.1 | 2.69 ± 0.09 |
| HPβ-CD | ||||
| 15 | 26 ± 2 | 1.53 ± 0.03 | 6.2 ± 0.2 | 1.51 ± 0.04 |
| 25 | 22 ± 1 | 1.63 ± 0.05 | 5.3 ± 0.2 | 1.72 ± 0.04 |
| 35 | 18 ± 1 | 1.85 ± 0.05 | 4.5 ± 0.3 | 2.02 ± 0.08 |
| 45 | 15 ± 1 | 2.22 ± 0.05 | 3.7 ± 0.1 | 2.58 ± 0.09 |
| Cyclodextrin | pH | ΔH (kJ mol−1) | ΔS (J mol−1 K−1) |
|---|---|---|---|
| β-CD | 2.8 | −26 ± 2 | −22 ± 6 |
| 7.0 | −16 ± 2 | −0.12 ± 2 | |
| Meβ-CD | 2.8 | −10 ± 1 | 34 ± 4 |
| 7.0 | −8.9 ± 1 | 19 ± 5 | |
| HPβ-CD | 2.8 | −14 ± 1 | 16 ± 2 |
| 7.0 | −13 ± 1 | 7 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zornoza, A.; Vélaz, I.; González-Gaitano, G.; Martínez-Ohárriz, M.C. A Comprehensive Study of Gemfibrozil Complexation with β-Cyclodextrins in Aqueous Solution Using Different Analytical Techniques. Int. J. Mol. Sci. 2022, 23, 16119. https://doi.org/10.3390/ijms232416119
Zornoza A, Vélaz I, González-Gaitano G, Martínez-Ohárriz MC. A Comprehensive Study of Gemfibrozil Complexation with β-Cyclodextrins in Aqueous Solution Using Different Analytical Techniques. International Journal of Molecular Sciences. 2022; 23(24):16119. https://doi.org/10.3390/ijms232416119
Chicago/Turabian StyleZornoza, Arantza, Itziar Vélaz, Gustavo González-Gaitano, and María Cristina Martínez-Ohárriz. 2022. "A Comprehensive Study of Gemfibrozil Complexation with β-Cyclodextrins in Aqueous Solution Using Different Analytical Techniques" International Journal of Molecular Sciences 23, no. 24: 16119. https://doi.org/10.3390/ijms232416119