Porous Crosslinked Zwitterionic Microparticles Based on Glycidyl Methacrylate and N-Vinylimidazole as Possible Drug Delivery Systems
Abstract
:1. Introduction
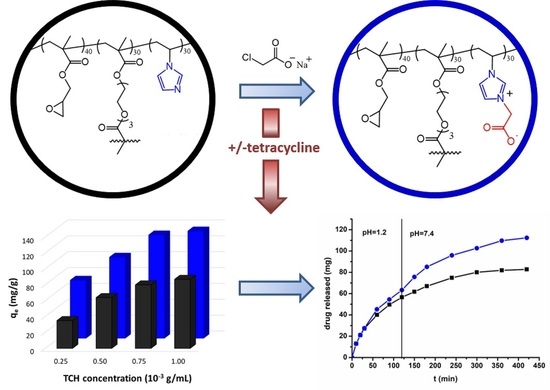
2. Results and Discussion
- GMA, a monomer with low toxicity and cheaper cost than other acrylic monomers, having two important functional groups in its chemical structure (methacrylic and epoxy groups), which may participate in polymerization or in ring opening reactions with the introduction of new functional groups [33,34];
- NVI, a monomer that may present biocompatibility, biodegradability and antibacterial activity, having an imidazole ring with a sterically unrestricted tertiary nitrogen atom, which in the presence of suitable betainization agents, via polymer–analogous reactions, lead to polymers containing betaine units [35,36];
- TEGDMA, a dimethacrylic monomer that can participate in crosslinking radical polymerization reactions due to the presence of the two double bonds in its chemical structure.
2.1. Antibacterial Activity
2.2. Tetracycline Loading
2.3. Tetracycline Release
- If n < 0.43, it corresponds to a release controlled by a diffusion process, indicating that the diffusion rate is much lower than that corresponding to the relaxation of the polymer chains. The mechanism of drug release is known as the Fick diffusion mechanism;
- If 0.43 < n < 0.85, it corresponds to an anomalous or non-Fickian diffusion mechanism where relaxation and diffusion rates have comparable values;
- If n > 0.85, the release mechanism is controlled by the relaxation phenomenon of the polymer chains and is known as super case II transport mechanism [45].
3. Materials and Methods
3.1. Materials
3.2. Synthesis Methods
3.2.1. Synthesis of Porous Crosslinked Microparticles
3.2.2. Synthesis of Porous Zwitterionic Microparticles
3.3. Characterization Methods
3.3.1. FT-IR Spectroscopy
3.3.2. SEM and EDAX
3.3.3. DVS Measurements
3.3.4. Mercury Intrusion Porosimetry
3.3.5. Swelling Degree
3.4. Antibacterial Activity
3.5. Adsorption and Release Studies of TCH
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kundawala, A.; Patel, V.; Patel, H.; Choundhary, D. Preparation in vitro characterization and in vivo pharmacokinetic evaluation of respirable porous microparticles containing rifampicin. Sci. Pharm. 2014, 82, 665–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Heasman, P.; Ben, T.; Qiu, S. Porous organic materials: Strategic design and structure-function-correlation. Chem. Rev. 2017, 117, 1515–1563. [Google Scholar] [CrossRef] [PubMed]
- Talha-Gokmen, M.; Du Prez, F.E. Porous polymer particles-A comprehensive guide to synthesis, characterization, functionalization and applications. Prog. Polym. Sci. 2012, 37, 365–405. [Google Scholar] [CrossRef] [Green Version]
- Dastidar Ghosh, D.; Saha, S.; Chowdhury, M. Porous microspheres: Synthesis, characterization and applications in pharmaceutical & medical fields. Int. J. Pharm. 2018, 548, 34–48. [Google Scholar]
- Dhamecha, D.; Le, D.; Movsas, R.; Gonsalves, A.; Munon, J.U. Porous polymeric microspheres with controllable pore diameters for tissue engineering lung tumor model development. Front. Bioeng. Biotechnol. 2020, 8, 799. [Google Scholar] [CrossRef]
- Peretz, S.; Anghel, D.F.; Vasilescu, E.; Florea-Spiroiu, M.; Stoian, C.; Zgherea, G. Synthesis, characterization and adsorption properties of alginate-porous beads. Polym. Bull. 2015, 72, 3169–3182. [Google Scholar] [CrossRef]
- Bai, Y.X.; Li, Y.F. Preparation and characterization of crosslinked porous cellulose beads. Carbohydr. Polym. 2006, 64, 402–407. [Google Scholar] [CrossRef]
- Rahmatpour, A.; Goodarzi, N.; Moazzez, M. A novel route for synthesis of crosslinked polystyrene copolymer beads with tunable porosity using guar and xanthan gums from bioresources as alternative synthetic suspension stabilizers. Des. Monom. Polym. 2018, 21, 116–129. [Google Scholar] [CrossRef] [Green Version]
- Gelli, R.; Mugnaini, G.; Bolognesi, T.; Bonini, M. Cross-linked porous gelatin microparticles with tunable shape, size and porosity. Langmuir 2021, 37, 12781–12789. [Google Scholar] [CrossRef]
- Quadrado, R.F.N.; Fajardo, A.R. Microparticles based on carboxymethyl starch/chitosan polyelectrolyte complex as vehicles for drug delivery systems. Arabian J. Chem. 2020, 13, 2183–2194. [Google Scholar] [CrossRef]
- Dowding, P.J.; Goodwin, J.W.; Vincent, B. Production of porous suspension polymer beads with a narrow size distribution using a cross-flow membrane and a continuous tubular reactor. Colloids Surf. A 2001, 180, 301–309. [Google Scholar] [CrossRef]
- Gupta, D.C.; Beldar, A.G.; Tank, R. Suspension copolymerization of styrene and divinylbenzene: Formation of beads. J. Appl. Polym. Sci. 2006, 101, 3559–3563. [Google Scholar] [CrossRef]
- Holcapkova, P.; Hrabalikova, M.; Stoplova, P.; Sedlarik, V. Core-shell PLA-PVA porous microparticles as carriers for bacteriocin nisin. J. Microencapsul. 2017, 34, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Peterson, T.E.; Gigliobianco, G.; Sherborne, C.; Green, N.H.; Dugan, J.M.; MacNeil, S.; Reilly, G.C.; Claeyssens, F. Porous microspheres support mesemchymal progenitor cell ingrowth and stimulate angiogenesis. APL Bioeng. 2018, 2, 026103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurapati, R.; Raichur, A.M. Composite cyclodextrin-calcium carbonate porous microparticles and modified multilayer capsules: Novel carriers for encapsulation of hydrophobic drugs. J. Mater. Chem. B 2013, 1, 3175–3184. [Google Scholar] [CrossRef] [PubMed]
- Paschke, S.; Lienkamp, K. Polyzwitterions: From surface properties and bioactivity profiles to biomedical applications. ACS Appl. Polym. Mater. 2020, 2, 129–151. [Google Scholar] [CrossRef]
- Khatoon, S.; Han, S.H.; Lee, M.; Lee, H.; Jung, D.W.; Thambi, T.; Ikram, M.; Kang, Y.M.; Yi, G.R.; Park, J.H. Zwitterionic mesoporous nanoparticles with a bioresponsive gatekeeper for cancer therapy. Acta Biomater. 2016, 40, 282–292. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Zhu, Y.H.; Wang, X.Y.; Chao, S.; Wei, X.W.; Xu, T.; He, Z.Y. Novel zwitterionic vectors: Multifunctional delivery system for therapeutic genes and drugs. Comput. Struct. Biotechnol. J. 2020, 18, 1980–1999. [Google Scholar] [CrossRef]
- Chou, Y.N.; Wen, T.C.; Chang, Y. Zwitterionic surface grafting of epoxylated sulfobetaine copolymers for development of stealth biomaterials interfaces. Acta Biomater. 2016, 40, 78–91. [Google Scholar] [CrossRef]
- Mi, L.; Jiang, S. Synchronizing nonfouling and antimicrobial properties in a zwitterionic hydrogels. Biomaterials 2012, 33, 8928–8933. [Google Scholar] [CrossRef]
- Geibel, C.; Dittrich, K.; Woiwode, U.; Kohout, M.; Zhang, T.; Lindner, W.; Lammerhofer, M. Evaluation of superficially porous particle based zwitterionic chiral ion exchangers against fully porous particles benchmarks for enantioselective ultra-high performance liquid chromatography. J. Chromat. A 2019, 1603, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Santi, M.; Emondts, M.; Roth, H.; Rahimi, K.; Großkurth, J.; Ganguly, R.; Wessling, M.; Singha, N.K.; Pich, A. Stimuli-responsive zwitterionic core-shell microgels for antifouling surface coatings. ACS Appl. Mater. Interfaces 2020, 12, 58223–58238. [Google Scholar] [CrossRef] [PubMed]
- Kitano, H.; Kawasaki, A.; Kawasaki, H.; Morokoshi, S. Resistance of zwitterionic telomers accumulated on metal surfaces against nonspecific adsorption of proteins. J. Colloid. Interface Sci. 2005, 282, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Kibar, G.; Tuncel, A. Synthesis and characterization of monodisperse-porous zwitterionic microbeads. Polym. Bull. 2016, 73, 1939–1950. [Google Scholar] [CrossRef]
- Liu, J.; Yang, K.; Shao, W.; Li, S.; Wu, Q.; Zhang, S.; Qu, Y.; Zhang, L.; Zhang, Y. Synthesis of zwitterionic polymer particles via combined distillation precipitation polymerization and click chemistry for high efficient enrichment of glycopeptide. ACS Appl. Mater. Interfaces 2016, 8, 22018–22024. [Google Scholar] [CrossRef] [PubMed]
- Neagu, V.; Vasiliu, S.; Racovita, S. Adsorption studies of some inorganic and organic salts on new zwitterionic ion exchangers with carboxybetaine moieties. Chem. Eng. J. 2010, 162, 965–973. [Google Scholar] [CrossRef]
- Zaharia, M.M.; Vasiliu, A.-L.; Trofin, M.A.; Pamfil, D.; Bucatariu, F.; Racovita, S.; Mihai, M. Design of multifunctional composite materials based on acrylic ion exchangers and CaCO3 as sorbents for small organic molecules. React. Funct. Polym. 2021, 166, 104997. [Google Scholar] [CrossRef]
- Zaharia, M.; Bucatariu, F.; Vasiliu, A.L.; Mihai, M. Stable and reusable acrylic ion-exchangers. From HMIs highly polluted tailing pond to safe and clean water. Chemosphere 2022, 304, 135383. [Google Scholar] [CrossRef]
- Zaharia, M.M.; Ghiorghita, C.-A.; Trofin, M.A.; Doroftei, F.; Rosca, I.; Mihai, M. Multifunctional composites of zwitterionic resins and silver nanoparticles for point-of-demand antimicrobial applications. Mater. Chem. Phys. 2022, 275, 125225. [Google Scholar] [CrossRef]
- Bale, S.; Khurana, A.; Reddy, A.S.S.; Singh, M.; Godugu, C. Overview on therapeutic applications of microparticulate drug delivery systems. Crit. Rev. Therapeut. Drug Carr. Syst. 2016, 33, 309–361. [Google Scholar] [CrossRef]
- Lengyel, M.; Kallai-Szabo, N.; Antal, V.; Laki, A.J.; Antal, I. Micropaticles, microspheres and microxapsules for advanced drug delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef]
- Racovita, S.; Trofin, M.-A.; Loghin, D.F.; Zaharia, M.-M.; Bucatariu, F.; Mihai, M.; Vasiliu, S. Polybetaines in biomedical applications. Int. J. Mol. Sci. 2021, 22, 9321. [Google Scholar] [CrossRef] [PubMed]
- Muzammil, E.Z.; Khan, A.; Stuparu, M.C. Post-polymerization modification reactions of poly(glycidyl methacrylate)s. RSC Adv. 2017, 7, 55874. [Google Scholar] [CrossRef] [Green Version]
- Cardil, A.; Palenzuela, M.; Vega, J.F.; Mosquera, M.E.G. Rheology of poly(glycidyl methacrylate) macromolecular nanoassemblies. Polymers 2022, 14, 455. [Google Scholar] [CrossRef] [PubMed]
- Caner, H.; Yilmaz, E.; Yilmaz, O. Synthesis, characterization and antibacterial activity of poly(N-vinylimidazole) grafted chitosan. Carbohydr. Polym. 2007, 69, 318–325. [Google Scholar] [CrossRef]
- Racovita, S.; Vasiliu, S.; Neagu, V. Solution properties of three polyzwitterions based on poly(N-vinylimidazole). Iran Polym. J. 2010, 19, 333–341. [Google Scholar]
- Wu, M.; Johannesson, B.; Geiker, M. A study of the water vapor sorption isotherms of hardebed cement pastes: Possible pore structure changes at low relative humidity and the impact of temperature on isotherms. Cem. Concr. Res. 2014, 56, 97–105. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Poulopoulos, S.G.; Kazemian, H. Insights into the S-shaped sorption isotherms and their dimensionless forms. Microporous Mesoporous Mater. 2018, 272, 166–176. [Google Scholar] [CrossRef]
- Cychosz, K.A.; Thommes, M. Progress in the physisorption characterization of nanoporous gas storage materials. Engineering 2018, 4, 559–566. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Patil, S.; Shettigar, H.; Bairwa, K.; Jana, S. Spectroscopic characterization of chloramphenicol and tetracycline: An impact of biofield treatment. Pharmaceut. Anal. Acta 2015, 6, 395. [Google Scholar]
- Varma, M.V.S.; Kaushal, A.M.; Garg, A.; Garg, S. Factors affecting mechanism and kinetics of drug release from matrix-based oral controlled drug delivery system. Am. J. Drug Deliv. 2004, 2, 43–57. [Google Scholar] [CrossRef]
- Higuchi, W.I. Diffusional models useful in biopharmaceutics. Drug release rate processes. J. Pharm. Sci. 1967, 56, 315–324. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar] [PubMed]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Zhan, S.; Wang, J.; Wang, W.; Cui, L.; Zhao, Q. Preparation and in vitro release kinetics of nitrendipine-loaded PLLA-PEG-PLLA microparticles by supercritical solution impregnation process. RSC Adv. 2019, 9, 16167. [Google Scholar] [CrossRef] [Green Version]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Yang, W.W.; Pierstorff, E. Reservoir-based polymer drug delivery systems. J. Lab. Autom. 2012, 17, 50–58. [Google Scholar] [CrossRef]
- Kun, E.; Marossy, K. Evaluation methods of antimicrobial activity of plastics. Mater. Sci. Forum 2013, 729, 430–435. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Shi, J.L.; Li, Y.S.; Chen, H.R.; Shen, W.H.; Dong, X.P. Storage and release of ibuprofen drug molecules in hollow mesoporous silica spheres with modified pore surface. Microporous Mesoporous Mater. 2005, 85, 75–81. [Google Scholar] [CrossRef]










| Sample Code | Specific Surface Area Ssp (m2/g) | Pore Volume (mL/g) | Porosity (%) | Mean Pore Diameter (nm) | Swelling Degree (%) | |
|---|---|---|---|---|---|---|
| Hg | DVS | |||||
| G40N30T30 | 48.32 | 289.13 | 1.314 | 46 | 109 | 261 |
| G40N30T30-ZW | 85.80 | 395.40 | 1.837 | 51 | 86 | 378 |
| Sample Codes | Higuchi Model | Korsmeyer–Peppas Model | Baker–Lonsdale Model | ||||
|---|---|---|---|---|---|---|---|
| kH (min−1/2) | R2 | kr (min−1/2) | n | R2 | kBL × 103 (min−1/2) | R2 | |
| G40N30T30-TCH | 0.092 | 0.982 | 0.040 | 0.609 | 0.994 | 1.170 | 0.992 |
| G40N30T30-ZW-TCH | 0.118 | 0.986 | 0.026 | 0.644 | 0.995 | 0.805 | 0.996 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trofin, M.-A.; Racovita, S.; Vasiliu, S.; Vasiliu, A.-L.; Mihai, M. Porous Crosslinked Zwitterionic Microparticles Based on Glycidyl Methacrylate and N-Vinylimidazole as Possible Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 14999. https://doi.org/10.3390/ijms232314999
Trofin M-A, Racovita S, Vasiliu S, Vasiliu A-L, Mihai M. Porous Crosslinked Zwitterionic Microparticles Based on Glycidyl Methacrylate and N-Vinylimidazole as Possible Drug Delivery Systems. International Journal of Molecular Sciences. 2022; 23(23):14999. https://doi.org/10.3390/ijms232314999
Chicago/Turabian StyleTrofin, Marin-Aurel, Stefania Racovita, Silvia Vasiliu, Ana-Lavinia Vasiliu, and Marcela Mihai. 2022. "Porous Crosslinked Zwitterionic Microparticles Based on Glycidyl Methacrylate and N-Vinylimidazole as Possible Drug Delivery Systems" International Journal of Molecular Sciences 23, no. 23: 14999. https://doi.org/10.3390/ijms232314999