Flagellar Phenotypes Impact on Bacterial Transport and Deposition Behavior in Porous Media: Case of Salmonella enterica Serovar Typhimurium
Abstract
:1. Introduction
2. Results
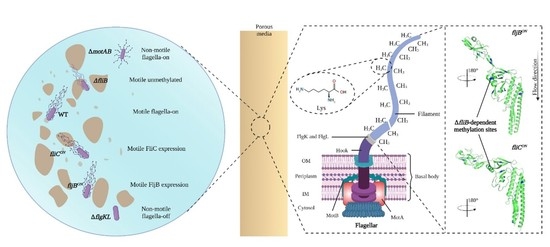
2.1. Flagella and Their Motility Impact Transport and Deposition Behavior of S. Typhimurium Strain in Sandy Porous Media
2.2. Flagella Phases Influence Transport and Deposition Behavior of Bacteria in Porous Media
2.3. Effects of Flagella Methylation on Transport and Deposition of Bacteria in Porous Media
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Column Transport Experiments
4.3. Simulation
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WWAP; UN-Water. World Water Development Report, Nature-Based Solutions for Water; UNESCO: Paris, France, 2018. [Google Scholar]
- Clemens, M.; Khurelbaatar, G.; Merz, R.; Siebert, C.; van Afferden, M.; Rödiger, T. Groundwater protection under water scarcity; from regional risk assessment to local wastewater treatment solutions in Jordan. Sci. Total Environ. 2020, 706, 136066. [Google Scholar] [CrossRef] [PubMed]
- Chrysikopoulos, C.V.; Sim, Y. One-dimensional virus transport in homogeneous porous media with time-dependent distribution coefficient. J. Hydrol. 1996, 185, 199–219. [Google Scholar] [CrossRef]
- Holden, N.; Pritchard, L.; Toth, I. Colonization outwith the colon: Plants as an alternative environmental reservoir for human pathogenic enterobacteria. FEMS Microbiol. Rev. 2009, 33, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Whitehouse, C.A.; Li, B. Presence and persistence of Salmonella in water: The impact on microbial quality of water and food safety. Front. Public Health 2018, 6, 159. [Google Scholar] [CrossRef] [PubMed]
- Abulreesh, H.H. Salmonellae in the environment. In Salmonella-Distribution, Adaptation, Control Measures and Molecular Technologies; Annous, B., Gurtler, J., Eds.; InTech: Rijeka, Croatia, 2012; pp. 19–50. [Google Scholar]
- Ma, H.; Bolster, C.; Johnson, W.P.; Li, K.; Pazmino, E.; Camacho, K.M.; Anselmo, A.C.; Mitragotri, S. Coupled influences of particle shape, surface property and flow hydrodynamics on rod-shaped colloid transport in porous media. J. Colloid. Interf. Sci. 2020, 577, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; McLeod, M.; Aislabie, J.; Šimůnek, J.; Close, M.; Hector, R. Modeling transport of microbes in ten undisturbed soils under effluent irrigation. Vadose Zone J. 2008, 7, 97–111. [Google Scholar] [CrossRef] [Green Version]
- Safadoust, A.; Mahboubi, A.A.; Mosaddeghi, M.R.; Gharabaghi, B.; Unc, A.; Voroney, P.; Heydari, A. Effect of regenerated soil structure on unsaturated transport of Escherichia coli and bromide. J. Hydrol. 2012, 430–431, 80–90. [Google Scholar] [CrossRef]
- Bai, H.; Cochet, N.; Drelich, A.; Pauss, A.; Lamy, E. Comparison of transport between two bacteria in saturated porous media with distinct pore size distribution. RSC Adv. 2016, 6, 14602–14614. [Google Scholar] [CrossRef]
- Ma, H.; Johnson, W.P. Colloid retention in porous media of various porosities: Predictions by the hemispheres-in-cell model. Langmuir 2010, 26, 1680–1687. [Google Scholar] [CrossRef]
- Prédélus, D.; Lassabatere, L.; Louis, C.; Gehan, H.; Brichart, T.; Winiarski, T.; Angulo-Jaramillo, R. Nanoparticle Transport in water-unsaturated porous media: Effects of solution ionic strength and flow rate. J. Nanopart. Res. 2017, 19, 104. [Google Scholar] [CrossRef]
- Yang, L.; Kang, J.; Chen, X.; Ripp, S.A.; Johnson, W.P.; Zhuang, J. Real-time bioluminescent imaging of spatiotemporal variation of microbial retention during transport through porous media under variably saturated flow conditions. J. Hydrol. 2021, 601, 126603. [Google Scholar] [CrossRef]
- Torkzaban, S.; Tazehkand, S.S.; Walker, S.L.; Bradford, S.A. Transport and fate of bacteria in porous media: Coupled effects of chemical conditions and pore space geometry. Water Resour. Res. 2008, 44, W04403. [Google Scholar] [CrossRef]
- Weiss, T.H.; Mills, A.L.; Hornberger, G.M.; Herman, J.S. Effect of bacterial cell shape on transport of bacteria in porous media. Environ. Sci. Technol. 1995, 29, 1737–1740. [Google Scholar] [CrossRef] [PubMed]
- Horstmann, J.A.; Lunelli, M.; Cazzola, H.; Heidemann, J.; Kühne, C.; Steffen, P.; Szefs, S.; Rossi, C.; Lokareddy, R.K.; Wang, C.; et al. Methylation of Salmonella Typhimurium flagella promotes bacterial adhesion and host cell invasion. Nat. Commun. 2020, 11, 2013. [Google Scholar] [CrossRef] [Green Version]
- Becker, M.W.; Collins, S.A.; Metge, D.W.; Harvey, R.W.; Shapiro, A.M. Effect of cell physicochemical characteristics and motility on bacterial transport in groundwater. J. Contam. Hydrol. 2004, 69, 195–213. [Google Scholar] [CrossRef] [PubMed]
- Josenhans, C.; Suerbaum, S. The role of motility as a virulence factor in bacteria. Int. J. Med. Microbiol. 2002, 291, 605–614. [Google Scholar] [CrossRef]
- Chen, S.; Beeby, M.; Murphy, G.E.; Leadbetter, J.R.; Hendrixson, D.R.; Briegel, A.; Li, Z.; Shi, J.; Tocheva, E.I.; Müller, A.; et al. Structural diversity of bacterial flagellar motors. EMBO J. 2011, 30, 2972–2981. [Google Scholar] [CrossRef] [Green Version]
- Vladimirov, N.; Sourjik, V. Chemotaxis: How bacteria use memory. Biol. Chem. 2009, 390, 1097–1104. [Google Scholar] [CrossRef]
- Zhang, M.; He, L.; Jin, X.; Bai, F.; Tong, M.; Ni, J. Flagella and their properties affect the transport and deposition behaviors of Escherichia coli in quartz sand. Environ. Sci. Technol. 2021, 55, 4964–4973. [Google Scholar] [CrossRef]
- Haznedaroglu, B.Z.; Zorlu, O.; Hill, J.E.; Walker, S.L. Identifying the role of flagella in the transport of motile and nonmotile Salmonella enterica Serovars. Environ. Sci. Technol. 2010, 44, 4184–4190. [Google Scholar] [CrossRef]
- Lederberg, J.; Iino, T. Phase Variation in Salmonella. Genetics 1956, 41, 743–757. [Google Scholar] [CrossRef] [PubMed]
- Horstmann, J.A.; Zschieschang, E.; Truschel, T.; de Diego, J.; Lunelli, M.; Rohde, M.; May, T.; Strowig, T.; Stradal, T.; Kolbe, M.; et al. Flagellin phase-dependent swimming on epithelial cell surfaces contributes to productive Salmonella gut colonisation. Cell Microbiol. 2017, 19, e12739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambler, R.P.; Rees, M.W. ε-N-Methyl-Lysine in Bacterial Flagellar Protein. Nature 1959, 184, 56–57. [Google Scholar] [CrossRef]
- Laasik, E.; Põllumaa, L.; Pasanen, M.; Mattinen, L.; Pirhonen, M.; Mäe, A. Expression of NipP.w of Pectobacterium wasabiae is dependent on functional FlgKL flagellar genes. Microbiology 2014, 160, 179–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, H.; Cochet, N.; Pauss, A.; Lamy, E. Bacteria cell properties and grain size impact on bacteria transport and deposition in porous media. Colloids Surf. B Biointerf. 2016, 139, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Cochet, N.; Pauss, A.; Lamy, E. DLVO, Hydrophobic, capillary and hydrodynamic forces acting on bacteria at solid-air-water interfaces: Their relative impact on bacteria deposition mechanisms in unsaturated porous media. Colloids Surf. B Biointerf. 2017, 150, 41–49. [Google Scholar] [CrossRef]
- Friedlander, R.S.; Vogel, N.; Aizenberg, J. Role of flagella in adhesion of Escherichia coli to abiotic surfaces. Langmuir 2015, 31, 6137–6144. [Google Scholar] [CrossRef]
- Cazzola, H.; Lemaire, L.; Acket, S.; Prost, E.; Duma, L.; Erhardt, M.; Čechová, P.; Trouillas, P.; Mohareb, F.; Rossi, C.; et al. The impact of plasma membrane lipid composition on flagellum-mediated adhesion of enterohemorrhagic Escherichia coli. mSphere 2020, 5, e00702-20. [Google Scholar] [CrossRef]
- Rossez, Y.; Wolfson, E.B.; Holmes, A.; Gally, D.L.; Holden, N.J. Bacterial flagella: Twist and stick, or dodge across the kingdoms. PLoS Pathog. 2015, 11, e1004483. [Google Scholar] [CrossRef]
- Rossez, Y.; Holmes, A.; Wolfson, E.B.; Gally, D.L.; Mahajan, A.; Pedersen, H.L.; Willats, W.G.T.; Toth, I.K.; Holden, N.J. Flagella interact with ionic plant lipids to mediate adherence of pathogenic Escherichia coli to fresh produce plants. Environ. Microbiol. 2014, 16, 2181–2195. [Google Scholar] [CrossRef]
- Jindai, K.; Nakade, K.; Masuda, K.; Sagawa, T.; Kojima, H.; Shimizu, T.; Shingubara, S.; Ito, T. Adhesion and bactericidal properties of nanostructured surfaces dependent on bacterial motility. RSC Adv. 2020, 10, 5673–5680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewangan, N.K.; Conrad, J.C. Bacterial motility enhances adhesion to oil droplets. Soft Matter 2020, 16, 8237–8244. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.-E.; He, L.; Heo, J.; Hwang, G. Implication of surface properties, bacterial motility, and hydrodynamic conditions on bacterial surface sensing and their initial adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef]
- Chevance, F.F.V.; Hughes, K.T. Coordinating assembly of a bacterial macromolecular machine. Nat. Rev. Microbiol. 2008, 6, 455–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cummings, L.A.; Wilkerson, W.D.; Bergsbaken, T.; Cookson, B.T. In Vivo, FliC expression by Salmonella enterica Serovar Typhimurium is heterogeneous, regulated by ClpX, and anatomically restricted. Mol. Microbiol. 2006, 61, 795–809. [Google Scholar] [CrossRef]
- Chaban, B.; Hughes, H.V.; Beeby, M. The flagellum in bacterial pathogens: For motility and a whole lot more. Semin. Cell Dev. Biol. 2015, 46, 91–103. [Google Scholar] [CrossRef] [Green Version]
- Barken, K.B.; Pamp, S.J.; Yang, L.; Gjermansen, M.; Bertrand, J.J.; Klausen, M.; Givskov, M.; Whitchurch, C.B.; Engel, J.N.; Tolker-Nielsen, T. Roles of type IV pili, flagellum-mediated motility and extracellular DNA in the formation of mature multicellular structures in Pseudomonas aeruginosa biofilms. Environ. Microbiol. 2008, 10, 2331–2343. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Friedlander, R.S.; Vlamakis, H.; Kim, P.; Khan, M.; Kolter, R.; Aizenberg, J. Bacterial flagella explore microscale hummocks and hollows to increase adhesion. Proc. Natl. Acad. Sci. USA 2013, 110, 5624–5629. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Ford, R.M.; Smith, J.A. Idling time of motile bacteria contributes to retardation and dispersion in sand porous medium. Environ. Sci. Technol. 2011, 45, 3945–3951. [Google Scholar] [CrossRef]
- Camper, A.K.; Hayes, J.T.; Sturman, P.J.; Jones, W.L.; Cunningham, A.B. Effects of motility and adsorption rate coefficient on transport of bacteria through saturated porous media. Appl. Environ. Microbiol. 1993, 59, 3455–3462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, N.; Bevard, T.; Massoudieh, A.; Zhang, C.; Dohnalkova, A.C.; Zilles, J.L.; Nguyen, T.H. Flagella-mediated differences in deposition dynamics for Azotobacter vinelandii in porous media. Environ. Sci. Technol. 2013, 47, 5162–5170. [Google Scholar] [CrossRef] [PubMed]
- de Kerchove, A.J.; Elimelech, M. Bacterial swimming motility enhances cell deposition and surface coverage. Environ. Sci. Technol. 2008, 42, 4371–4377. [Google Scholar] [CrossRef]
- Misselwitz, B.; Barrett, N.; Kreibich, S.; Vonaesch, P.; Andritschke, D.; Rout, S.; Weidner, K.; Sormaz, M.; Songhet, P.; Horvath, P.; et al. Near surface swimming of Salmonella Typhimurium explains target-site selection and cooperative invasion. PLoS Pathog. 2012, 8, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunstan, J.; Miño, G.; Clement, E.; Soto, R. A Two-sphere model for bacteria swimming near solid surfaces. Phys. Fluids 2012, 24, 011901. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, T.; Toma, S.; Terahara, N.; Miyata, T.; Ashihara, M.; Minamino, T.; Namba, K.; Kato, T. Structural and functional comparison of Salmonella flagellar filaments composed of FljB and FliC. Biomolecules 2020, 10, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolster, C.H.; Walker, S.L.; Cook, K.L. Comparison of Escherichia coli and Campylobacter jejuni transport in saturated porous media. J. Environ. Qual. 2006, 35, 1018–1025. [Google Scholar] [CrossRef] [Green Version]
- Eregno, F.E.; Tryland, I.; Tjomsland, T.; Myrmel, M.; Robertson, L.; Heistad, A. Quantitative microbial risk assessment combined with hydrodynamic modelling to estimate the public health risk associated with bathing after rainfall events. Sci. Total Environ. 2016, 548–549, 270–279. [Google Scholar] [CrossRef]
- Tolouei, S.; Dewey, R.; Snodgrass, W.J.; Edge, T.A.; Andrews, R.C.; Taghipour, M.; Prévost, M.; Dorner, S. Assessing microbial risk through event-based pathogen loading and hydrodynamic modelling. Sci. Total Environ. 2019, 693, 133567. [Google Scholar] [CrossRef]
- Sokolova, E.; Petterson, S.R.; Dienus, O.; Nyström, F.; Lindgren, P.-E.; Pettersson, T.J.R. Microbial risk assessment of drinking water based on hydrodynamic modelling of pathogen concentrations in source water. Sci. Total Environ. 2015, 526, 177–186. [Google Scholar] [CrossRef]
- Bradford, S.A.; Simunek, J.; Walker, S.L. Transport and straining of E. coli O157:H7 in saturated porous media. Water Resour. Res. 2006, 42. [Google Scholar] [CrossRef] [Green Version]
- Sanders, E.R. Aseptic laboratory techniques: Plating methods. J. Vis. Exp. 2012, 63, e3064. [Google Scholar] [CrossRef] [PubMed]
- Sardin, M.; Schweich, D.; Leij, F.J.; van Genuchten, M.T. Modeling the nonequilibrium transport of linearly interacting solutes in porous media: A review. Water Resour. Res. 1991, 27, 2287–2307. [Google Scholar] [CrossRef]
- Lamy, E.; Lassabatere, L.; Bechet, B.; Andrieu, H. Effect of a nonwoven geotextile on solute and colloid transport in porous media under both saturated and unsaturated conditions. Geotext. Geomembr. 2013, 36, 55–65. [Google Scholar] [CrossRef]
- Bradford, S.A.; Simunek, J.; Bettahar, M.; van Genuchten, M.T.; Yates, S.R. Modeling colloid attachment, straining, and exclusion in saturated porous media. Environ. Sci. Technol. 2003, 37, 2242–2250. [Google Scholar] [CrossRef]





| Flagellar Phenotypes | Wild Type | ΔflgKL | ΔmotAB | fljBON | fliCON | ΔfliB |
|---|---|---|---|---|---|---|
| EM774 | EM4969 | EM4939 | EM1013 | EM1012 | EM3734 | |
| Cell motility | Motile | Non-motile | Non-motile | Motile | Motile | Motile |
| Flagella | On | Off | On | On | On | On |
| Bacterial Strains | Replicate | Saturated Degree (%) | C Initial (CFU/mL) | Porosity (%) | Bulk Density (g/cm3) | Pulse Time (min) | Darcy Velocity (cm/min) | Retardation Factor | Recovery (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Meff | Mretained | Mtotal | |||||||||
| WT | 1 | 98.2 | 7.60 × 108 | 37.88 | 1.65 | 2.40 | 0.906 | 0.94 | 51.97 | 34.64 | 86.61 |
| 2 | 98.5 | 1.40 × 109 | 36.41 | 1.69 | 2.33 | 0.936 | 0.79 | 46.49 | 26.99 | 73.48 | |
| 3 | 98.1 | 6.40 × 108 | 37.98 | 1.64 | 2.37 | 0.939 | 0.86 | 42.83 | 27.35 | 70.18 | |
| (0.86) a | (47.1) | (29.66) | (76.76) | ||||||||
| ΔflgKL | 1 | 98.4 | 1.27 × 109 | 38.92 | 1.62 | 2.20 | 1.010 | 0.66 | 44.41 | 17.69 | 62.10 |
| 2 | 98.9 | 1.23 × 109 | 38.26 | 1.64 | 1.85 | 1.191 | 0.66 | 44.83 | 22.41 | 67.24 | |
| 3 | 99.1 | 1.57 × 109 | 37.91 | 1.65 | 2.23 | 0.991 | 0.65 | 44.87 | 29.69 | 74.56 | |
| (0.66) | (44.7) | (23.26) | (67.97) | ||||||||
| 0.42 b | 0.16 | 0.18 | |||||||||
| ΔmotAB | 1 | 96.4 | 1.30 × 109 | 37.68 | 1.65 | 2.32 | 0.951 | 0.98 | 38.78 | 31.20 | 69.98 |
| 2 | 97.9 | 1.73 × 109 | 37.17 | 1.66 | 2.44 | 0.903 | 0.99 | 38.45 | 25.37 | 63.82 | |
| 3 | 96.6 | 1.93 × 109 | 37.77 | 1.65 | 2.67 | 0.826 | 0.75 | 33.12 | 22.81 | 55.93 | |
| (0.91) | (36.78) | (26.46) | (63.24) | ||||||||
| 0.03 | 0.42 | 0.10 | |||||||||
| ΔfliB | 1 | 98.4 | 9.00 × 108 | 37.92 | 1.65 | 2.36 | 0.934 | 1.01 | 44.8 | 20.93 | 65.73 |
| 2 | 97.3 | 1.60 × 109 | 35.12 | 1.72 | 2.11 | 1.045 | 1.04 | 46.25 | 8.36 | 54.61 | |
| 3 | 99.5 | 9.33 × 108 | 38.06 | 1.64 | 2.22 | 0.994 | 0.75 | 42.89 | 13.48 | 56.37 | |
| (0.93) | (44.65) | (14.26) | (58.9) | ||||||||
| 0.44 | 0.03 | 0.04 | |||||||||
| fljBON | 1 | 98.6 | 1.17 × 109 | 36.84 | 1.67 | 2.07 | 1.066 | 0.79 | 40.44 | 33.58 | 74.02 |
| 2 | 99.2 | 9.00 × 108 | 36.79 | 1.67 | 2.34 | 0.941 | 0.70 | 38.34 | 17.76 | 56.10 | |
| 3 | 98.8 | 1.00 × 109 | 36.91 | 1.67 | 2.36 | 0.935 | 0.84 | 38.98 | 14.46 | 53.44 | |
| (0.77) | (39.25) | (21.93) | (61.18) | ||||||||
| 0.04 | 0.29 | 0.13 | |||||||||
| fliCON | 1 | 93.5 | 1.57 × 109 | 36.46 | 1.68 | 2.15 | 1.025 | 0.72 | 51.02 | 24.54 | 75.56 |
| 2 | 96.0 | 1.03 × 109 | 36.09 | 1.69 | 2.33 | 0.945 | 0.65 | 46.28 | 17.53 | 63.81 | |
| 3 | 100.0 | 1.10 × 109 | 36.38 | 1.69 | 2.27 | 0.972 | 0.86 | 46.90 | 13.70 | 60.60 | |
| (0.74) | (48.07) | (18.59) | (66.66) | ||||||||
| 0.77 | 0.05 | 0.2 | |||||||||
| Bacterial Strains | Replicate | λ (cm) | θm/θ | Katt (min−1) | Kd (min−1) | Kstr (min−1) | R2 |
|---|---|---|---|---|---|---|---|
| WT | 1 | 0.69 | 0.54 | 3.12 × 10−2 | 3.93 × 10−1 | 7.87 × 10−6 | 0.9963 |
| (0.06) * | (0.004) | (1.58 × 10−2) | (2.10 × 10−1) | (6.56 × 10−3) | |||
| 2 | 0.48 | 0.51 | 1.46 × 10−1 | 6.07 × 10−1 | 8.35 × 10−2 | 0.9958 | |
| (0.07) | (0.008) | (1.88 × 10−2) | (6.09 × 10−2) | (1.25 × 10−1) | |||
| 3 | 0.29 | 0.52 | 8.59 × 10−2 | 4.29 × 10−1 | 1.85 × 10−1 | 0.9963 | |
| (0.03) | (0.002) | (1.36 × 10−2) | (8.16 × 10−2) | (2.49 × 10−2) | |||
| Mean values | 0.49 | 0.53 | 0.09 | 0.48 | 0.09 | 0.996 | |
| (0.2001) ** | 0.0166 | 0.0574 | 0.1147 | 0.0929 | 0.0003 | ||
| ΔflgKL | 1 | 0.35 | 0.62 | 3.25 × 10−2 | 3.38 × 10−1 | 1.76 × 10−1 | 0.9987 |
| (0.03) | (0.002) | (5.00 × 10−3) | (1.17 × 10−1) | (6.23 × 10−2) | |||
| 2 | 0.21 | 0.58 | 1.23 × 10−1 | 8.76 × 10−1 | 1.71 × 10−1 | 0.9968 | |
| (0.04) | (0.004) | (1.82 × 10−2) | (1.71 × 10−1) | (8.46 × 10−2) | |||
| 3 | 0.29 | 0.65 | 2.68 × 10−2 | 4.99 × 10−1 | 1.71 × 10−1 | 0.9948 | |
| (0.05) | (0.003) | (2.52 × 10−2) | (9.80 × 10−1) | (6.95 × 10−2) | |||
| Mean values | 0.28 | 0.62 | 0.06 | 0.57 | 0.17 | 0.997 | |
| 0.0699 | 0.0375 | 0.0539 | 0.2761 | 0.0029 | 0.0020 | ||
| ΔmotAB | 1 | 0.39 | 0.52 | 1.35 × 10−1 | 3.80 × 10−1 | 2.18 × 10−1 | 0.9793 |
| (0.14) | (0.03) | (3.95 × 10−2) | (7.83 × 10−2) | (3.14 × 10−1) | |||
| 2 | 0.55 | 0.53 | 9.22 × 10−2 | 2.63 × 10−1 | 2.03 × 10−1 | 0.9668 | |
| (0.20) | (0.04) | (4.15 × 10−2) | (1.37 × 10−1) | (4.68 × 10−1) | |||
| 3 | 0.21 | 0.64 | 4.93 × 10−2 | 5.72 × 10−1 | 4.63 × 10−1 | 0.9969 | |
| (0.02) | (0.002) | (5.48 × 10−3) | (1.37 × 10−1) | (5.45 × 10−2) | |||
| Mean values | 0.38 | 0.56 | 0.09 | 0.40 | 0.29 | 0.981 | |
| 0.1703 | 0.0654 | 0.0428 | 0.1560 | 0.1459 | 0.0151 | ||
| ΔfliB | 1 | 0.14 | 0.67 | 3.37 × 10−1 | 5.63 × 10−1 | 1.03 × 10−1 | 0.9823 |
| (0.04) | (0.02) | (1.83 × 10−2) | (5.74 × 10−2) | (1.12 × 10−1) | |||
| 2 | 0.30 | 0.51 | 1.36 × 10−1 | 5.74 × 10−1 | 5.82 × 10−2 | 0.9852 | |
| (0.13) | (0.02) | (5.11 × 10−2) | (1.33 × 10−1) | (3.21 × 10−1) | |||
| 3 | 0.34 | 0.56 | 7.50 × 10−2 | 4.42 × 10−1 | 2.00 × 10−1 | 0.9989 | |
| (0.03) | (0.003) | (4.58 × 10−3) | (4.35 × 10−2) | (5.62 × 10−2) | |||
| Mean values | 0.26 | 0.58 | 0.18 | 0.53 | 0.12 | 0.989 | |
| 0.1042 | 0.0852 | 0.1368 | 0.0732 | 0.0726 | 0.0088 | ||
| fliCON | 1 | 0.32 | 0.65 | 2.99 × 10−2 | 6.92 × 10−1 | 1.69 × 10−4 | 0.9945 |
| (0.06) | (0.005) | (3.91 × 10−2) | (9.65 × 10−1) | (4.69 × 10−2) | |||
| 2 | 0.59 | 0.40 | 2.87 × 10−1 | 8.25 × 10−1 | 1.57 × 10−1 | 0.9958 | |
| (0.16) | (0.008) | (1.09 × 10−1) | (1.78 × 10−1) | (3.92 × 10−2) | |||
| 3 | 0.35 | 0.59 | 6.01 × 10−2 | 5.37 × 10−1 | 5.01 × 10−2 | 0.9988 | |
| (0.02) | (0.002) | (1.17 × 10−2) | (1.06 × 10−1) | (1.47 × 10−2) | |||
| Mean values | 0.42 | 0.54 | 0.13 | 0.68 | 0.07 | 0.996 | |
| 0.1496 | 0.1295 | 0.1407 | 0.1441 | 0.0804 | 0.0022 | ||
| fljBON | 1 | 0.22 | 0.51 | 1.64 × 10−1 | 7.61 × 10−1 | 3.60 × 10−1 | 0.9985 |
| (0.02) | (0.002) | (2.10 × 10−2) | (8.43 × 10−2) | (2.04 × 10−2) | |||
| 2 | 0.76 | 0.54 | 1.14 × 10−1 | 5.29 × 10−1 | 3.86 × 10−1 | 0.9947 | |
| (0.24) | (0.02) | (6.74 × 10−2) | (2.03 × 10−1) | (2.07 × 10−1) | |||
| 3 | 0.33 | 0.59 | 7.71 × 10−2 | 6.00 × 10−1 | 2.68 × 10−1 | 0.9972 | |
| (0.04) | (0.005) | (6.61 × 10−3) | (9.98 × 10−2) | (1.00 × 10−1) | |||
| Mean values | 0.44 | 0.54 | 0.12 | 0.63 | 0.34 | 0.997 | |
| 0.2885 | 0.0416 | 0.0436 | 0.1185 | 0.0621 | 0.0019 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Bai, H.; Tao, Y.; Achak, M.; Rossez, Y.; Lamy, E. Flagellar Phenotypes Impact on Bacterial Transport and Deposition Behavior in Porous Media: Case of Salmonella enterica Serovar Typhimurium. Int. J. Mol. Sci. 2022, 23, 14460. https://doi.org/10.3390/ijms232214460
Zheng X, Bai H, Tao Y, Achak M, Rossez Y, Lamy E. Flagellar Phenotypes Impact on Bacterial Transport and Deposition Behavior in Porous Media: Case of Salmonella enterica Serovar Typhimurium. International Journal of Molecular Sciences. 2022; 23(22):14460. https://doi.org/10.3390/ijms232214460
Chicago/Turabian StyleZheng, Xin, Hongjuan Bai, Ye Tao, Mounia Achak, Yannick Rossez, and Edvina Lamy. 2022. "Flagellar Phenotypes Impact on Bacterial Transport and Deposition Behavior in Porous Media: Case of Salmonella enterica Serovar Typhimurium" International Journal of Molecular Sciences 23, no. 22: 14460. https://doi.org/10.3390/ijms232214460