Peripheral Gonadotropin-Inhibitory Hormone (GnIH) Acting as a Novel Modulator Involved in Hyperphagia-Induced Obesity and Associated Disorders of Metabolism in an In Vivo Female Piglet Model
Abstract
:1. Introduction
2. Results
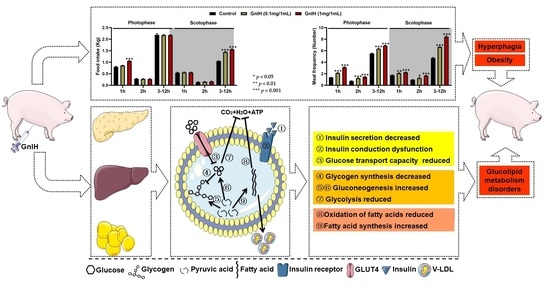
2.1. Intraperitoneally Injected GnIH Increases Piglets Food Intake and Alters Meal Microstructure
2.2. Intraperitoneally Injected GnIH Decreases Feed/Gain Ratio and Increases Pig Obesity
2.3. Intraperitoneally Injected GnIH Influence Serum Biochemical Indexes and Alters Body Fat Composition
2.4. Effects of Intraperitoneally Injected GnIH on Glucose Homeostasis and Islet Histomorphology Changes for Investigated
2.5. Effect of Intraperitoneally Injected GnIH on the Expression Levels of Hepatic Glucometabolism-Related Genes
2.6. Effect of Intraperitoneally Injected GnIH on the Expression Levels of Glucometabolism-Related Genes in WAT
2.7. Effect of Intraperitoneally Injected GnIH on the Expression Levels of Hepatic Lipid Metabolism-Related Genes
2.8. Effect of Intraperitoneally Injected GnIH on the Expression Levels of Lipid Metabolism-Related Genes in WAT
3. Discussion
4. Materials and Methods
4.1. Animals and GnIH
4.2. Animal Treatments
4.2.1. Grouping
4.2.2. Injections Protocol
4.2.3. Fasting Treatments
4.3. Food Intake, Meal Microstructure and Weight Measurements
4.4. Porcine Obesity Index
4.5. Serum and Tissue Collection
4.6. Serum Biochemical Analyses
4.7. Blood Glucose Measurements
4.8. Glucose Tolerance Test
4.9. Gene Expression
4.10. Western Blot Analysis
4.11. Morphological Analysis
4.12. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pastore, A.P.; Cesaretti, M.L.; Ginoza, M.; Voltera, A.F.; Junior, O.K. Effects of the association of experimental neuroendocrine and exocrine obesity on tail blood pressure and glucose metabolism in Wistar rats. Braz. J. Nephrol. 2010, 32, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Nejat, E.J.; Polotsky, A.J.; Pal, L. Predictors of chronic disease at midlife and beyond--the health risks of obesity. Maturitas 2010, 65, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Lockie, S.H. Glucagon-like peptide-1 receptor in the brain: Role in neuroendocrine control of energy metabolism and treatment target for obesity. J. Neuroendocrinol. 2013, 25, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Tena-Sempere, M. Neuroendocrinology in 2016: Neuroendocrine control of metabolism and reproduction. Nat. Rev. Endocrinol. 2017, 13, 67–68. [Google Scholar] [CrossRef] [PubMed]
- Muccioli, G.; Tschop, M.; Papotti, M.; Deghenghi, R.; Heiman, M.; Ghigo, E. Neuroendocrine and peripheral activities of ghrelin: Implications in metabolism and obesity. Eur. J. Pharmacol. 2002, 440, 235–254. [Google Scholar] [CrossRef]
- Tolson, K.P.; Garcia, C.; Yen, S.; Simonds, S.; Stefanidis, A.; Lawrence, A.; Smith, J.T.; Kauffman, A.S. Impaired kisspeptin signaling decreases metabolism and promotes glucose intolerance and obesity. J. Clin. Investig. 2014, 124, 3075–3079. [Google Scholar] [CrossRef] [Green Version]
- Tsutsui, K.; Saigoh, E.; Ukena, K.; Teranishi, H.; Fujisawa, Y.; Kikuchi, M.; Ishii, S.; Sharp, P.J. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem. Biophys. Res. Commun. 2000, 275, 661–667. [Google Scholar] [CrossRef]
- Yoshida, H.; Habata, Y.; Hosoya, M.; Kawamata, Y.; Kitada, C.; Hinuma, S. Molecular properties of endogenous RFamide-related peptide-3 and its interaction with receptors. Biochim. Biophys. Acta Mol. Cell Res. 2003, 1593, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Ubuka, T.; Morgan, K.; Pawson, A.J.; Osugi, T.; Chowdhury, V.S.; Minakata, H.; Tsutsui, K.; Millar, R.P.; Bentley, G.E. Identification of human GnIH homologs, RFRP-1 and RFRP-3, and the cognate receptor, GPR147 in the human hypothalamic pituitary axis. PLoS ONE 2009, 4, e8400. [Google Scholar] [CrossRef] [Green Version]
- Ukena, K.; Iwakoshi, E.; Minakata, H.; Tsutsui, K. A novel rat hypothalamic RFamide-related peptide identified by immunoaffinity chromatography and mass spectrometry. FEBS Lett. 2002, 512, 255–258. [Google Scholar] [CrossRef]
- Li, X.; Su, J.; Lei, Z.; Zhao, Y.; Jin, M.; Fang, R.; Zheng, L.; Jiao, Y. Gonadotropin-inhibitory hormone (GnIH) and its receptor in the female pig: cDNA cloning, expression in tissues and expression pattern in the reproductive axis during the estrous cycle. Peptides 2012, 36, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, T.; Sato, M.; Takahashi, H.; Ukena, K.; Tsutsui, K.; Furuse, M. Gonadotropin-inhibiting hormone stimulates feeding behavior in chicks. Brain Res. 2005, 1050, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.A.; Tsutsui, K.; Fraley, G.S. Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Horm. Behav. 2007, 51, 171–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McConn, B.; Wang, G.; Yi, J.; Gilbert, E.R.; Osugi, T.; Ubuka, T.; Tsutsui, K.; Chowdhury, V.S.; Furuse, M.; Cline, M.A. Gonadotropin-inhibitory hormone-stimulation of food intake is mediated by hypothalamic effects in chicks. Neuropeptides 2014, 48, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Huo, K.; Li, X.; Hu, W.; Song, X.; Zhang, D.; Zhang, X.; Chen, X.; Yuan, J.; Zuo, J.; Wang, X. RFRP-3, the Mammalian Ortholog of GnIH, Is a Novel Modulator Involved in Food Intake and Glucose Homeostasis. Front. Endocrinol. 2020, 11, 194. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Chen, L.; Song, X.; Zhang, X.; Xu, W.; Han, D.; Zuo, J.; Hu, W.; Shi, Y.; Cao, Y.; et al. Possible Role of GnIH as a Novel Link between Hyperphagia-Induced Obesity-Related Metabolic Derangements and Hypogonadism in Male Mice. Int. J. Mol. Sci. 2022, 23, 8066. [Google Scholar] [CrossRef]
- Hill, J.O.; Wyatt, H.R.; Peters, J.C. Energy balance and obesity. Circulation 2012, 126, 126–132. [Google Scholar] [CrossRef]
- Andreoli, M.F.; Stoker, C.; Rossetti, M.F.; Alzamendi, A.; Castrogiovanni, D.; Luque, E.H.; Ramos, J.G. Withdrawal of dietary phytoestrogens in adult male rats affects hypothalamic regulation of food intake, induces obesity and alters glucose metabolism. Mol. Cell. Endocrinol. 2015, 401, 111–119. [Google Scholar] [CrossRef]
- Anjum, S.; Krishna, A.; Tsutsui, K. Possible Role of GnIH as a Mediator between Adiposity and Impaired Testicular Function. Front. Endocrinol. 2016, 7, 6. [Google Scholar] [CrossRef] [Green Version]
- Moriwaki, S.; Narimatsu, Y.; Fukumura, K.; Iwakoshi-Ukena, E.; Furumitsu, M.; Ukena, K. Effects of Chronic Intracerebroventricular Infusion of RFamide-Related Peptide-3 on Energy Metabolism in Male Mice. Int. J. Mol. Sci. 2020, 21, 8606. [Google Scholar] [CrossRef]
- Roura, E.; Koopmans, S.J.; Lalles, J.P.; le Huerou-Luron, I.; de Jager, N.; Schuurman, T.; Val-Laillet, D. Critical review evaluating the pig as a model for human nutritional physiology. Nutr. Res. Rev. 2016, 29, 60–90. [Google Scholar] [CrossRef]
- Bergen, W.G. Pigs (Sus Scrofa) in Biomedical Research. Recent Adv. Anim. Nutr. Metab. 2022, 1354, 335–343. [Google Scholar]
- Baker, D.H. Animal models in nutrition research. J. Nutr. 2008, 138, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Anjum, S.; Khattak, M.N.K.; Tsutsui, K.; Krishna, A. RF-amide related peptide-3 (RFRP-3): A novel neuroendocrine regulator of energy homeostasis, metabolism, and reproduction. Mol. Biol. Rep. 2021, 48, 1837–1852. [Google Scholar] [CrossRef] [PubMed]
- McConn, B.R.; Yi, J.; Gilbert, E.R.; Siegel, P.B.; Chowdhury, V.S.; Furuse, M.; Cline, M.A. Stimulation of food intake after central administration of gonadotropin-inhibitory hormone is similar in genetically selected low and high body weight lines of chickens. Gen. Comp. Endocrinol. 2016, 232, 96–100. [Google Scholar] [CrossRef]
- Clarke, I.J.; Smith, J.T.; Henry, B.A.; Oldfield, B.J.; Stefanidis, A.; Millar, R.P.; Sari, I.P.; Chng, K.; Fabre-Nys, C.; Caraty, A.; et al. Gonadotropin-inhibitory hormone is a hypothalamic peptide that provides a molecular switch between reproduction and feeding. Neuroendocrinology 2012, 95, 305–316. [Google Scholar] [CrossRef] [PubMed]
- van Erp, R.J.J.; de Vries, S.; van Kempen, T.A.T.G.; den Hartog, L.A.; Gerrits, W.J.J. Circadian misalignment imposed by nocturnal feeding tends to increase fat deposition in pigs. Br. J. Nutr. 2019, 123, 529–536. [Google Scholar] [CrossRef]
- Schneider, J.D.; Tokach, M.D.; Dritz, S.S.; Nelssen, J.L.; Derouchey, J.M.; Goodband, R.D. Effects of feeding schedule on body condition, aggressiveness, and reproductive failure in group-housed sows. J. Anim. Sci. 2007, 85, 3462–3469. [Google Scholar] [CrossRef]
- Carco, G.; Gallo, L.; Bona, M.D.; Latorre, M.A.; Fondevila, M.; Schiavon, S. The influence of feeding behaviour on growth performance, carcass and meat characteristics of growing pigs. PLoS ONE 2018, 13, e0205572. [Google Scholar] [CrossRef] [Green Version]
- Cazarez-Marquez, F.; Eliveld, J.; Ritsema, W.; Foppen, E.; Bossenbroek, Y.; Pelizzari, S.; Simonneaux, V.; Kalsbeek, A. Role of central kisspeptin and RFRP-3 in energy metabolism in the male Wistar rat. J. Neuroendocrinol. 2021, 33, e12973. [Google Scholar] [CrossRef]
- Davidson, M.B. Pathogenesis of impaired glucose tolerance and type II diabetes mellitus—current status. West. J. Med. 1985, 142, 219–229. [Google Scholar] [PubMed]
- Zhang, W.; Wang, L.; Yu, X.; Jia, A.; Ming, J.; Ji, Q. RFamide-related peptide-3 promotes alpha TC1 clone 6 cell survival likely via GPR. Peptides 2018, 107, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Warner, S.O.; Wadian, A.M.; Smith, M.; Farmer, B.; Dai, Y.; Sheanon, N.; Edgerton, D.S.; Winnick, J.J. Liver glycogen-induced enhancements in hypoglycemic counterregulation require neuroglucopenia. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E914–E924. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Huang, T.; Zhen, X.; Li, Y.; Mo, M.; Ye, D.; Cheng, N. A selective sphingomyelin synthase 2 inhibitor ameliorates diet induced insulin resistance via the IRS-1/Akt/GSK-3beta signaling pathway. Pharmazie 2019, 74, 553–558. [Google Scholar] [PubMed]
- Yang, K.; Chen, Z.; Gao, J.; Shi, W.; Li, L.; Jiang, S.; Hu, H.; Liu, Z.; Xu, D.; Wu, L. The Key Roles of GSK-3beta in Regulating Mitochondrial Activity. Cell. Physiol. Biochem. 2017, 44, 1445–1459. [Google Scholar] [CrossRef] [PubMed]
- Lowell, B.B.; Shulman, G.I. Mitochondrial dysfunction and type 2 diabetes. Science 2005, 307, 384–387. [Google Scholar] [CrossRef] [Green Version]
- Titchenell, P.M.; Lazar, M.A.; Birnbaum, M.J. Unraveling the Regulation of Hepatic Metabolism by Insulin. Trends Endocrinol. Metab. 2017, 28, 497–505. [Google Scholar] [CrossRef]
- Klover, P.J.; Mooney, R.A. Hepatocytes: Critical for glucose homeostasis. Int. J. Biochem. Cell Biol. 2004, 36, 753–758. [Google Scholar] [CrossRef]
- Konner, A.C.; Bruning, J.C. Selective insulin and leptin resistance in metabolic disorders. Cell Metab. 2012, 16, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Liu, S.; Zhai, A.; Zhang, B.; Tian, G. AMPK-Mediated Regulation of Lipid Metabolism by Phosphorylation. Biol. Pharm. Bull. 2018, 41, 985–993. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Zhou, X.; Huang, N.; Li, H.; Tian, J.; Li, T.; Yao, K.; Nyachoti, C.M.; Kim, S.W.; Yin, Y. AMPK Regulation of Glucose, Lipid and Protein Metabolism: Mechanisms and Nutritional Significance. Curr. Protein Pept. Sci. 2017, 18, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Viollet, B. The Energy Sensor AMPK: Adaptations to Exercise, Nutritional and Hormonal Signals. In Hormones, Metabolism and the Benefits of Exercise; Research and Perspectives in Endocrine Interactions; Springer: Cham, Switzerland, 2017; pp. 13–24. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.T.; Kwon, D.Y.; Yoon, S.H. AMP-activated protein kinase: A potential target for the diseases prevention by natural occurring polyphenols. New Biotechnol. 2009, 26, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Malley, C.O.; Pidgeon, G.P. The mTOR pathway in obesity driven gastrointestinal cancers: Potential targets and clinical trials. BBA Clin. 2016, 5, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Munday, M.R. Regulation of mammalian acetyl-CoA carboxylase. Biochem. Soc. Trans. 2002, 30, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Mitro, N.; Mak, P.A.; Vargas, L.; Godio, C.; Hampton, E.; Molteni, V.; Kreusch, A.; Saez, E. The nuclear receptor LXR is a glucose sensor. Nature 2007, 445, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Guillemet, R.; Dourmad, J.Y.; Meunier-Salaun, M.C. Feeding behavior in primiparous lactating sows: Impact of a high-fiber diet during pregnancy. J. Anim. Sci. 2006, 84, 2474–2481. [Google Scholar] [CrossRef] [PubMed]
- Tolkamp, B.J.; Allcroft, D.J.; Barrio, J.P.; Bley, T.A.; Howie, J.A.; Jacobsen, T.B.; Morgan, C.A.; Schweitzer, D.P.; Wilkinson, S.; Yeates, M.P.; et al. The temporal structure of feeding behavior. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R378–R393. [Google Scholar] [CrossRef] [Green Version]
- Sebert, S.P.; Lecannu, G.; Kozlowski, F.; Siliart, B.; Bard, J.M.; Krempf, M.; Champ, M.M. Childhood obesity and insulin resistance in a Yucatan mini-piglet model: Putative roles of IGF-1 and muscle PPARs in adipose tissue activity and development. Int. J. Obes. 2005, 29, 324–333. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.; Honek, J.; Xue, Y.; Seki, T.; Cao, Z.; Andersson, P.; Yang, X.; Hosaka, K.; Cao, Y. Cold-induced activation of brown adipose tissue and adipose angiogenesis in mice. Nat. Protoc. 2012, 7, 606–615. [Google Scholar] [CrossRef]
- Zhao, C.; Sun, Q.; Tang, L.; Cao, Y.; Nourse, J.L.; Pathak, M.M.; Lu, X.; Yang, Q. Mechanosensitive Ion Channel Piezo1 Regulates Diet-Induced Adipose Inflammation and Systemic Insulin Resistance. Front. Endocrinol. 2019, 10, 373. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Hu, C. RFRP-3, the mammalian ortholog of GnIH, induces cell cycle arrest at G2/M in porcine ovarian granulosa cells. Peptides 2018, 101, 106–111. [Google Scholar] [CrossRef] [PubMed]








| Parameters | Photophase (12 h) | Scotophase (12 h) | ||||
|---|---|---|---|---|---|---|
| Control | GnIH | GnIH | Control | GnIH | GnIH | |
| (0 mg/1 mL) | (0.1 mg/1 mL) | (1 mg/1 mL) | (0 mg/1 ml) | (0.1 mg/1 mL) | (1 mg/1 mL) | |
| Food intake (kg/12 h) | 3.27 ± 0.02 | 3.31 ± 0.03 | 3.51 ± 0.02 *** | 1.73 ± 0.02 | 2.17 ± 0.01 *** | 2.27 ± 0.03 *** |
| Meal frequency (number/12 h) | 7.59 ± 0.15 | 9.67 ± 0.20 *** | 11.46 ± 0.19 *** | 7.41 ± 0.13 | 9.99 ± 0.16 *** | 12.3 ± 0.15 *** |
| Time spent in meals (min/12 h) | 137.9 ± 4.87 | 151.5 ± 3.97 * | 154.6 ± 1.85 ** | 121.8 ± 2.72 | 144.5 ± 2.27 *** | 152.7 ± 1.77 *** |
| Meal size (kg/meal) | 0.43 ± 0.01 | 0.34 ± 0.01 *** | 0.30 ± 0.01 *** | 0.23 ± 0.01 | 0.21 ± 0.01 * | 0.18 ± 0.01 *** |
| Eating rate (g/s) | 0.39 ± 0.02 | 0.37 ± 0.01 | 0.38 ± 0.01 | 0.24 ± 0.01 | 0.25 ± 0.01 | 0.25 ± 0.01 |
| Meal duration (min/meal) | 18.27 ± 0.29 | 15.67 ± 0.37 *** | 13.5 ± 0.25 *** | 16.40 ± 0.29 | 14.5 ± 0.29 *** | 12.4 ± 0.22 *** |
| Inter-meal interval (min) | 77.34 ± 1.32 | 58.79 ± 1.48 *** | 49.34 ± 0.86 *** | 80.67 ± 1.65 | 57.62 ± 1.02 *** | 46.03 ± 0.55 *** |
| Satiety ratio (min/kg) | 220.19 ± 1.46 | 217.32 ± 2.15 | 204.74 ± 0.99 *** | 416.22 ± 3.93 | 331.30 ± 1.90 *** | 316.78 ± 4.55 *** |
| Gnens | Primer sequence (5′-3′) | Genebank No. | Function |
|---|---|---|---|
| IRS1 | F:GAATCTCAGTCCCAACCGCAAC | NM_001244489.1 | Insulin signal transduction |
| R:CTGGGTGTCGAGGAGAAGGTCTC | |||
| IRS2 | F:ACAGACTAAATACAACGCACGACTC | XM_021065907.1 | Insulin signal transduction |
| R:GAAGTATATTTCTGGCTCTTGGGAC | |||
| LXRA | F:ATCCGCCTGAAGAAACTGAAGC | XM_013994348.2 | Cholesterol metabolism |
| R:CTGGTCTGAAAAGGAGCGTCTG | |||
| FBP1 | F:CTCTCCAATGACCTGGTTATTAACG | NM_213979.1 | Gluconeogenesis |
| R:TTTCTGTAGATGCCAAAGATGGTTC | |||
| PEPCK | F:TCAGCACGACTCCAGCCTTCA | NM_001123158.1 | Gluconeogenesis |
| R:GCTCAAGCAGTCTGGGCATTCT | |||
| GCK | F:ATCAAACGGAGAGGGGACTT | XM_013985832.2 | Glucose metabolism |
| R:ACAATCATGCCAACCTCACA | |||
| FASN | F:CTACGAGGCCATTGTGGACG | NM_001099930.1 | Fatty acid synthesis |
| R:AGCCTATCATGCTGTAGCCC | |||
| ACC | F:AGCAAGGTCGAGACCGAAG | NM_001114269.1 | Fatty acid synthesis |
| R:TAAGACCACCGGCGGATAGA | |||
| ACLY | F:GAGGCAGCATCGCAAACTTCA | XM_021066028.1 | Fatty acid transport |
| R:TCCCAACTTCTCCCATCACCC | |||
| ATP5B | F:GAATCCCTTCTGCGGTGGGTTAT | XM_001929410.5 | ATP synthase |
| R:GGCAGGAGCAGGGTCAGTCAAGT | |||
| GlUT-4 | F:GTATGTTGCGGATGCTATGGG | NM_001128433.1 | Glucose transporter |
| R:CTCGGGTTTCAGGCACTTTTAG | |||
| CPT-1 | F:TCACAAGCGAATTTGAGTGC | NM_001129805.1 | Fatty acid beta oxidation |
| R:AAATTCAGACCGCAGTTTCG | |||
| FABP4 | F:AGTGGGATGGAAAGACAACCAC | NM_001002817.1 | Fatty acid transport |
| R:GTCGGGACAATACATCCAACAG | |||
| AMPKα1 | F:TGTCACAGGCATATGGTGGTC | XM_021076522.1 | Energy metabolism |
| R:GGACCAGCATACAACCTTCCT | |||
| Glucagon | F:ACATTGCCAAACGTCACGATG | XM_005671883.3 | Glucagon synthesis |
| R:GCCTTCCTCGGCCTTTCA | |||
| Insulin | F:GCCTTTGTGAACCAACACCTG | XM_021081278.1 | Insulin synthesis |
| R:GTTGCAGTAGTTCTCCAGCTG | |||
| β-actin | F:TGGAACGGTGAAGGTGACAGC | XM_003124280.5 | Reference genes |
| R:GCTTTTGGGAAGGCAGGGACT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Zhang, X.; Song, X.; Han, D.; Han, K.; Xu, W.; Luo, R.; Cao, Y.; Shi, Y.; Liu, C.; et al. Peripheral Gonadotropin-Inhibitory Hormone (GnIH) Acting as a Novel Modulator Involved in Hyperphagia-Induced Obesity and Associated Disorders of Metabolism in an In Vivo Female Piglet Model. Int. J. Mol. Sci. 2022, 23, 13956. https://doi.org/10.3390/ijms232213956
Chen L, Zhang X, Song X, Han D, Han K, Xu W, Luo R, Cao Y, Shi Y, Liu C, et al. Peripheral Gonadotropin-Inhibitory Hormone (GnIH) Acting as a Novel Modulator Involved in Hyperphagia-Induced Obesity and Associated Disorders of Metabolism in an In Vivo Female Piglet Model. International Journal of Molecular Sciences. 2022; 23(22):13956. https://doi.org/10.3390/ijms232213956
Chicago/Turabian StyleChen, Lei, Xin Zhang, Xingxing Song, Dongyang Han, Kaiou Han, Wenhao Xu, Rongrong Luo, Yajie Cao, Yan Shi, Chengcheng Liu, and et al. 2022. "Peripheral Gonadotropin-Inhibitory Hormone (GnIH) Acting as a Novel Modulator Involved in Hyperphagia-Induced Obesity and Associated Disorders of Metabolism in an In Vivo Female Piglet Model" International Journal of Molecular Sciences 23, no. 22: 13956. https://doi.org/10.3390/ijms232213956