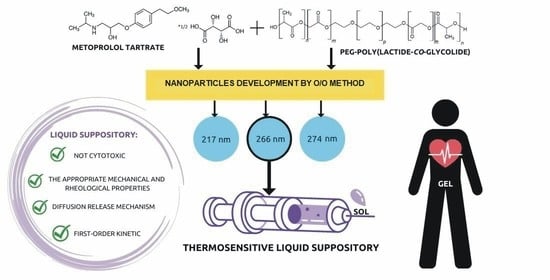
3.1. Materials
Glycolide (1,4-dioxane-2,5-dione, ≥99%, Sigma, Poznan, Poland), L,L-Lactide ((3S)-cis-3,6-dimethyl-1,4-dioxane-2,5-dione, 98.0%, Aldrich Co., Poznan, Poland), poly(ethylene glycol) 600 (PEG 600, pure, Fluka, Warsaw, Poland), ZnEt2 solution (15% diethylzinc in toluene, Aldrich Co., Poznan, Poland), hydrochloric acid (HCl, ChemPur, Piekary Slaskie, Poland), methanol (pure, 99.9%, ChemPur, Piekary Slaskie, Poland), dichloromethane (DCM, CH2CL2, ≥99.8%, POCh, Gliwice, Poland), chloroform (pure, 99%, ChemPur, Piekary Slaskie, Poland), metoprolol tartrate (>98.0%, TCI, Tokyo, Japan), phosphate-buffered saline (PBS, pH 7.00 ± 0.05, ChemPur, Piekary Slaskie, Poland), Kalliphor® P 188 (Poloxamer 188, Lutrol® F68, Aldrich Co., Poznan, Poland) Poloxamer 407 (Aldrich Co., Poznan, Poland), PVP (Mw 40,000, Aldrich Co., Poznan, Poland), Tween 80 (viscous liquid, Aldrich Co., Poznan, Poland), Sodium alginate (Aldrich Co., Poznan, Poland), HPC (TCI, Tokyo, Japan), Liquid paraffin (ChemPur, Piekary Slaskie, Poland), Trifluoroacetate acid (TFA, ≥98%, Sigma, Poznan, Poland), n-heksan (99%, POCh, Gliwice, Poland), stock solution of Zn(II) (concentration 1000 mg/L, Merck, Darmstadt, Germany), 65% nitric acid (HNO3, J.T. Baker, Deventer, Netherlands), dialysis membrane Spectra/Por 3 MWCO 3500 (Spectrum Laboratories, Inc., Gardena, CA, USA) were used as received.
3.2. Biodegradable PLGA Carrier
3.2.1. Synthesis Route
The copolymeric materials were synthesized by varying the molar ratios of the initiator (PEG 600) to the monomers: GA or L,L-lactide. For the synthesized polymers, the initiator/monomer feed ratios were 1/10/90 (mol/mol). PEG and monomers were precisely weighed and placed in a 50 mL polymerization tube. The tube was then linked to a Schlenk line, and the exhausting-refilling cycle was performed three times. The catalyst ZnEt2 was then added in the next step. The tube was then immersed in an oil bath at 120 °C for 48 h. After a period of time, the polymer products were cooled and dissolved in dry chloroform. The copolymer was then precipitated three times from cold methanol containing 5% hydrochloric acid (HCl). The organic phase was evaporated and dried in a vacuum for 48 h.
3.2.2. Microstructural Analysis
The structure of the obtained PLGA copolymers was elucidated by 1H and 13C NMR techniques. The spectra were recorded using Varian 300 MHz (Palo Alto, Santa Clara, CA, USA) and Agilent Technologies 400 MHz (Santa Clara, CA, USA) spectrometer. The microstructure of matrices was studied using 13C NMR and 1H NMR spectra of copolymer PLGA.
The LLA and GA conversions were calculated using the following formulae from the
1H NMR spectra of the post-reaction mixture:
where
Iα and
ID correspond to integral intensities of signals from methylene protons adjacent to α carbon atoms of
LLA/
GA monomer and
LLA/
GA units in PLGA copolymer, respectively [
25].
The average molecular weight (Mn) and dispersity index (Đ) of the obtained copolymer were measured by SEC-MALLS. Mn and Đ were measured using SEC-MALLS instrument (Wyatt Technology Corporation, Santa Barbara, CA, USA) composed of an 1100 Agilent isocratic pump, autosampler, degasser, thermostatic box for columns, a photometer MALLS DAWN EOS (Wyatt Technology Corporation, Santa Barbara, CA, USA) and differential refractometer Optilab Rex (Wyatt Technology Corporation, Santa Barbara, CA, USA). ASTRA 4.90.07 software (Wyatt Technology Corporation, Santa Barbara, CA, USA) was used for data collecting and processing. Two 2× TSKgel MultiporeHXL columns were used for separation. The samples were injected as a solution in methylene chloride. The volume of the injection loop was 100 mL. Methylene chloride was used as a mobile phase at a flow rate of 0.8 mL·min−1.
3.2.3. Spectroscopic Analysis
1H NMR (DMSO-d
6, 400 MHz, δH, ppm): 1.56 (PLA, –CH
3), 3.62 (PEG, -CH
2), 4.75 (PGL, –C(O)CH
2O–), 5.16 (PLA, –C(O)CH(CH
3)O–) (
Figure S1 (
1H NMR spectra of PEG-PLGA,
Supplementary Material).
13C NMR (CDCl
3, 300 MHz, δC, ppm): 169 (C=O), 166 (C=O), 70 (PEG, -CH
2-), 69 (PEG, -CH
2)), 66 (LA, -CH-), 64 (GA, -CH
2-), 16 (LA, -CH
3) (
Figure S2,
13C NMR spectra of PEG-PLGA,
Supplementary Material).
FTIR (KBr, cm
−1): 3509 (υ
O–H), 2993–2882 (υ
C-H), 1754 (υ
C=O), 1459–1385 υ
s(
C-H) u
as (
C-H), 1135 υ(
C-O-C) (
Figure S3, FT-IR spectra of PEG-PLGA,
Supplementary Material).
3.2.4. Determination of the Catalyst Residue
The detection of the catalyst residue (zinc ions) was carried out using a Perkin Elmer Analyst 400 flame atomic absorption spectrometer equipped with a hollow cathode lamp and a deuterium background corrector at the respective wavelengths using an air-acetylene flame and the manufacturer’s recommended instrumental parameters.
0.500 g of the dried sample was digested with 6.00 cm3 of concentrated HNO3 closed polytetrafluoroethylene (PTFE) vessels in a microwave oven (Multiwave 3000, Anton Paar (Perkin Elmer). After digestion the solution was transferred into the 100.0 cm3 volumetric flask and filled to the mark with Type I (ISO 3696) deionized water of resistivity > 10 MΩ·cm. Zinc contents were determined directly in respective solutions by FAAS with the air-acetylene flame and hollow cathode lamp as light source.
3.2.5. Biological Assays
The cyto- and genotoxicity experiments were performed on the produced copolymeric products in accordance with pharmacopoeia standards for biomaterials. The umu-test and the neutral red uptake test were used to measure the cytotoxicity and genotoxicity of the copolymers obtained.
The neutral red uptake test was performed on the basis of ISO 10993 guideline Annex A [
26] with BALB/c 3T3 clone A31 mammalian cell line (mouse embryonic fibroblasts from American Type Culture Collection). The quantitative estimation of viable cells in tested cultures was based on their neutral red uptake in comparison to the results obtained for untreated cells. Dead cells have no ability to accumulate the dye in their lysosomes.
The BALB/c 3T3 cells were seeded in 96-well microplates (15,000 cells/100 µL) in DMEM (Lonza) culture medium (supplemented with 10% of calf bovine serum, 100 IU/mL penicillin and 0.1 mg/mL streptomycin) and incubated for 24 h (5% CO2, 37 °C, >90% humidity). At the end of the incubation each well was examined under a microscope to ensure that cells form a confluent monolayer. After that culture medium was replaced by the tested extracts. Extracts were prepared by incubation of tested materials in the cell culture medium (1 mg/mL for polymers; 100 mg/mL for suppositories) with reduced serum concentration (5%) at 37 °C for 24 h with shaking and sterilizes by filtration. Cells were treated with four dilutions of each extract in a twofold dilution series for 24 h (three data points for each one). Subsequently treatment medium was removed. Cells were washed with PBS and treated with the neutral red medium for 2 h. Than the medium was discarded, cells were washed with PBS and treated with desorbing fixative (ethanol and acetic acid water solution). The amount of neutral red accumulated by cells were evaluated colorimetrically at 540 nm. Polyethylene film and latex were used as the reference materials (with no cytotoxicity and highly cytotoxic, respectively). At the same time, the impact of the metoprolol content in some samples on the test results was evaluated. The percentage of viable cells in each well was calculated by comparing its OD540 result with the mean result obtained for untreated cells (incubated in the same conditions with fresh culture medium). Samples were considered cytotoxic if they reduced cell survival below 70% compared to the untreated cells (a baseline cells viability). When the BALB/c 3T3 cells viability was not decreased under 70% in the whole range of tested dilutions of the samples, it was considered as non-cytotoxic in this range of concentrations.
The
umu-test was performed in 96-well microplates according to the ISO 13829 protocol [
27] with and without metabolic activation (S9 liver fraction, Xenometrix). Deionized sterile water was used as a negative control, 2-aminoanthracene and 4-nitroquinoline N-oxide were used as positive controls. All tested materials were incubated in phosphate buffered saline (PBS from Gibco) for 24 h, 37 °C with shaking. Before the assay all extracts were sterilized by filtration. All samples were tested in two fold dilution series (three concentrations, three points of date for each one). Clear PBS treated in the same way as all samples were tested as a solvent control.
3.5. In Vitro MT Release Studies
2 g of thermosensitive LSs-loaded-MT and 35 mg of thermosensitive LSs-based MT-loaded nanoparticles were injected into vials. The thermosensitive LSs samples were then thermostated at 37 °C for 5 min to form gels, and nanoparticles were suspended in 1 mL PBS buffer (pH = 7.00 ± 0.05). Following that, the materials were immersed in dialysis membrane (3500 Da), placed in 10.0 mL of preheated PBS buffer (pH = 7.00 ± 0.05), and shaken at 130 rpm and 37 °C. After predefined time intervals, the release medium was withdrawn for further testing and entirely replaced with 10.0 mL of preheated fresh PBS buffer. The obtained samples were stored at 18 °C prior to HPLC analysis. The drug release experiments lasted 12 h.
The HPLC apparatus (Beckman Coulter, Miami, Florida, USA) was equipped with an autosampler (Triathlon 900, Spark Holland B.V., Emmen, Netherlands), pomp (Beckman Coulter System Gold® 125NM Solvent Module, Fullerton, CA, USA) and UV/VIS detector (Beckman Coulter System Gold® 166, Fullerton, CA, USA). The analysis was performed at 274 nm with a C18 column (Nucleodur C18 Gravity 150 × 4.6 mm, 5 um, Macherey-Nagel). Solvents A and B (75:25), H2O + 0.05% TFA and acetonitrile (ACN), were used to make the mobile phase. The flow rate was set to 1.0 mL/min, the injection volume was set to 20 µL, and the column temperature was set to 35 °C.
Mathematic Models
The kinetics and mechanism of MT release were determined by fitting experimental data to theoretical mathematical models, zero-order and first-order, Higuchi and Korsmeyer–Peppas, using the following equations:
Korsmeyer-Peppas model:
where,
F—the amount of drug released;
F0—the initial concentration of MT,
t—the release time increment;
k—the model constant,
Mt/M∞—the fraction of MT released during time t;
n—the exponent in the Korsmeyer–Peppas model [
29,
30].