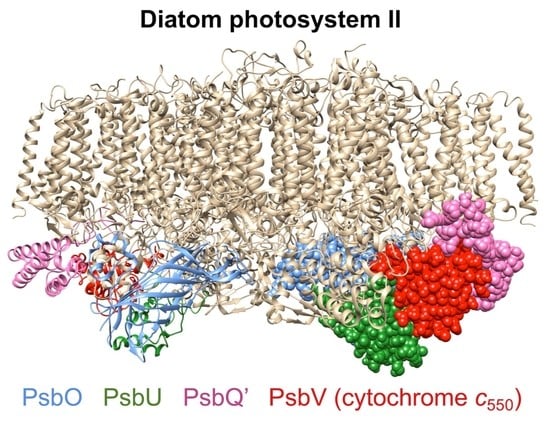
Iron Deficiency Promotes the Lack of Photosynthetic Cytochrome c550 and Affects the Binding of the Luminal Extrinsic Subunits to Photosystem II in the Diatom Phaeodactylum tricornutum
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Cell Growth
4.2. Protein Analysis Methods
4.3. Photosynthetic Measurements
4.4. Statistical Significance Level
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hervás, M.; Navarro, J.A.; De la Rosa, M.A. Electron transfer between membrane complexes and soluble proteins in photosynthesis. Acc. Chem. Res. 2003, 35, 785–805. [Google Scholar] [CrossRef] [PubMed]
- Morrisey, J.; Bowler, C. Iron utilization in marine cyanobacteria and eukaryotic algae. Front. Microbiol. 2012, 3, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kranzler, C.; Rudolf, M.; Keren, N.; Schleif, E. Iron in Cyanobacteria. In Genomics of Cyanobacteria; Chauvat, F., Cassier-Chauvat, C., Eds.; Advances in Botanical Research; Academic Press: San Diego, CA, USA, 2013; Volume 65, pp. 57–105. [Google Scholar]
- Geider, R.J.; La Roche, J.; Greene, R.M.; Olaizola, M. Response of the photosynthetic apparatus of Phaeodactylum tricornutum (Bacillariophyceae) to nitrate, phosphate, or iron starvation. J. Phycol. 1993, 29, 755–766. [Google Scholar] [CrossRef]
- Moore, J.K.; Doney, S.C.; Glover, D.M.; Fung, I.Y. Iron cycling and nutrient limitation patterns in surface waters of the world ocean. Deep Sea Res. Part II 2002, 49, 463–508. [Google Scholar] [CrossRef] [Green Version]
- Castell, C.; Rodríguez-Lumbreras, L.A.; Hervás, M.; Fernández-Recio, J.; Navarro, J.A. New insights into the evolution of the electron transfer from cytochrome f to photosystem I in the green and red branches of photosynthetic eukaryotes. Plant Cell Physiol. 2021, 62, 1082–1093. [Google Scholar] [CrossRef]
- Allen, A.E.; LaRoche, J.; Maheswari, U.; Lommer, M.; Schauer, N.; Lopez, P.J.; Finazzi, G.; Fernie, A.R.; Bowler, C. Whole-cell response of the pennate diatom Phaeodactylum tricornutum to iron starvation. Proc. Natl. Acad. Sci. USA 2008, 105, 10438–10443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunn, B.L.; Faux, J.F.; Hippmann, A.A.; Maldonado, M.T.; Harvey, H.R.; Goodlett, D.R.; Boyd, P.W.; Strzepek, R.F. Diatom proteomics reveals unique acclimation strategies to mitigate Fe limitation. PLoS ONE 2013, 8, e75653. [Google Scholar] [CrossRef] [Green Version]
- Roncel, M.; González-Rodríguez, A.A.; Naranjo, B.; Bernal-Bayard, P.; Lindahl, M.; Hervás, M.; Navarro, J.A.; Ortega, J.A. Iron deficiency induces a partial inhibition of the photosynthetic electron transport and a high sensitivity to light in the diatom Phaeodactylum tricornutum. Front. Plant Sci. 2016, 7, 1050. [Google Scholar] [CrossRef] [Green Version]
- Castell, C.; Bernal-Bayard, P.; Ortega, J.M.; Roncel, M.; Hervás, M.; Navarro, J.A. The heterologous expression of a plastocyanin in the diatom Phaeodactylum tricornutum improves cell growth under iron-deficient conditions. Physiol. Plant. 2021, 171, 277–290. [Google Scholar] [CrossRef]
- Shen, J.-R. The structure of photosystem II and the mechanism of water oxidation in photosynthesis. Annu. Rev. Plant Biol. 2015, 66, 23–48. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.-A.; Chiu, Y. The roles of cytochrome b559 in assembly and photoprotection of photosystem II revealed by site-directed mutagenesis studies. Front. Plant Sci. 2016, 6, 1261. [Google Scholar] [CrossRef] [Green Version]
- Msilini, N.; Zaghdoudi, M.; Govindachary, S.; Lachaâl, M.; Ouerghi, Z.; Carpentier, R. Inhibition of photosynthetic oxygen evolution and electron transfer from the quinone acceptor QA− to QB by iron deficiency. Photosynth. Res. 2011, 107, 247–256. [Google Scholar] [CrossRef]
- Müh, F.; Zouni, A. The nonheme iron in photosystem II. Photosynth. Res. 2013, 116, 295–314. [Google Scholar] [CrossRef]
- Enami, I.; Iwai, M.; Akiyama, A.; Suzuki, T.; Okumura, A.; Katoh, T.; Tada, O.; Ohta, H.; Shen, J.-R. Comparison of binding and functional properties of two extrinsic components, cyt c550 and a 12 kDa protein, in cyanobacterial PSII with those in red algal PSII. Plant Cell Physiol. 2003, 44, 820–827. [Google Scholar] [CrossRef] [Green Version]
- Nagao, R.; Moriguchi, A.; Tomo, T.; Niikura, A.; Nakajima, S.; Suzuki, T.; Okumura, A.; Iwai, M.; Shen, J.-R.; Ikeuchi, M.; et al. Binding and functional properties of five extrinsic proteins in oxygen-evolving photosystem II from a marine centric diatom, Chaetoceros gracilis. J. Biol. Chem. 2010, 285, 29191–29199. [Google Scholar] [CrossRef] [Green Version]
- Pi, X.; Zhao, S.; Wang, W.; Liu, D.; Xu, C.; Han, G.; Kuang, T.; Sui, S.-F.; Shen, J.-R. The pigment-protein network of a diatom photosystem II-light harvesting antenna supercomplex. Science 2019, 365, eaax4406. [Google Scholar] [CrossRef]
- Umena, Y.; Kawakami, K.; Shen, J.-R.; Kamiya, N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 2011, 473, 55–60. [Google Scholar] [CrossRef]
- Roncel, M.; Kirilovsky, D.; Guerrero, F.; Serrano, A.; Ortega, J.M. Photosynthetic cytochrome c550. Biochim. Biophys. Acta-Bioenerg. 2012, 1817, 1152–1163. [Google Scholar] [CrossRef] [Green Version]
- Enami, I.; Okumura, A.; Nagao, R.; Suzuki, T.; Iwai, M.; Shen, J.-R. Structures and functions of the extrinsic proteins of photosystem II from different species. Photosynth. Res. 2008, 98, 349–363. [Google Scholar] [CrossRef]
- Ago, H.; Adachi, H.; Umena, Y.; Tashiro, T.; Kawakami, K.; Kamiya, N.; Tian, L.; Han, G.; Kuang, T.; Liu, Z.; et al. Novel features of eukaryotic photosystem II revealed by its crystal structure analysis from a red alga. J. Biol. Chem. 2016, 291, 5676–5687. [Google Scholar] [CrossRef]
- Bernal-Bayard, P.; Puerto-Galán, L.; Yruela, I.; García-Rubio, I.; Castell, C.; Ortega, J.M.; Alonso, P.J.; Roncel, M.; Martínez, J.I.; Hervás, M.; et al. The photosynthetic cytochrome c550 from the diatom Phaeodactylum tricornutum. Photosynth. Res. 2017, 133, 273–287. [Google Scholar] [CrossRef] [Green Version]
- Ifuku, K.; Noguchi, T. Structural coupling of extrinsic proteins with the oxygen-evolving center in photosystem II. Front. Plant Sci. 2016, 7, 84. [Google Scholar] [CrossRef] [Green Version]
- Nagao, R.; Kato, K.; Suzuki, T.; Ifuku, K.; Uchiyama, I.; Kashino, Y.; Dohmae, N.; Akimoto, S.; Shen, J.-R.; Miyazaki, N.; et al. Structural basis for energy harvesting and dissipation in a diatom PSII-FCPII supercomplex. Nat. Plants 2019, 5, 890–901. [Google Scholar] [CrossRef]
- Shen, J.R.; Inoue, Y. Binding and functional properties of two new extrinsic components, cytochrome c550 and a 12-kDa protein, in cyanobacterial photosystem II. Biochemistry 1993, 32, 1825–1832. [Google Scholar] [CrossRef]
- Enami, I.; Kikuchi, S.; Fukuda, T.; Ohta, H.; Shen, J. Binding and functional properties of four extrinsic proteins of photosystem II from a red alga, Cyanidium caldarium, as studied by release-reconstitution experiments. Biochemistry 1998, 37, 2787–2793. [Google Scholar] [CrossRef]
- Shen, J.-R.; Qian, M.; Inoue, Y.; Burnap, R.L. Functional characterization of Synechocystis sp. PCC 6803 ΔpsbU and ΔpsbV mutants reveals important roles of cytochrome c-550 in cyanobacterial oxygen evolution. Biochemistry 1998, 37, 1551–1558. [Google Scholar] [CrossRef]
- Bricker, T.M.; Roose, J.L.; Fagerlund, R.D.; Frankel, L.K.; Eaton-Rye, J.J. The extrinsic proteins of Photosystem II. Biochim. Biophys. Acta-Bioenerg. 2012, 1817, 121–142. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Zhu, Q.; Yang, Y.; Wang, W.; Kuang, T.; Shen, J.-R.; Han, G. Role of PsbV-Tyr137 in photosystem II studied by site-directed mutagenesis in the thermophilic cyanobacterium Thermosynechococcus vulcanus. Photosynth. Res. 2020, 146, 41–54. [Google Scholar] [CrossRef]
- Kirilovsky, D.; Roncel, M.; Boussac, A.; Wilson, A.; Zurita, J.L.; Ducruet, J.M.; Bottin, H.; Sugiura, M.; Ortega, J.M.; Rutherford, A.W. Cytochrome c550 in the cyanobacterium Thermosynechococcus elongatus: Study of redox mutants. J. Biol. Chem. 2004, 279, 52869–52880. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Andrews, H.; Eaton-Rye, J.J.; Burnap, R.L. In situ effects of mutations of the extrinsic cytochrome c550 of photosystem II in Synechocystis sp. PCC6803. Biochemistry 2004, 43, 14161–14170. [Google Scholar] [CrossRef]
- Vogt, L.; Vinyard, D.J.; Khan, S.; Brudvig, G.W. Oxygen-evolving complex of Photosystem II: An analysis of second-shell residues and hydrogen-bonding networks. Curr. Opin. Chem. Biol. 2015, 25, 152–158. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.R.; Gillard, J.T.; Kustka, A.B.; McCrow, J.P.; Badger, J.H.; Zheng, H.; New, A.M.; Dupont, C.L.; Obata, T.; Fernie, A.R.; et al. Transcriptional Orchestration of the Global Cellular Response of a Model Pennate Diatom to Diel Light Cycling under Iron Limitation. PLoS Genet. 2016, 12, e1006490. [Google Scholar] [CrossRef] [Green Version]
- Ortega, J.M.; Roncel, M. The afterglow photosynthetic luminescence. Physiol. Plant. 2021, 171, 268–276. [Google Scholar] [CrossRef]
- Sane, P.V.; Ivanov, A.G.; Öquist, G.; Hüner, N.P.A. Thermoluminescence. In Photosynthesis: Plastid Biology, Energy Conversion and Carbon Assimilation; Advances in Photosynthesis and Respiration; Eaton-Rye, J.J., Tripathy, B.C., Sharkey, T.D., Eds.; Springer: Dordrecht, The Netherlands, 2012; Volume 34, Chapter 19; pp. 445–474. [Google Scholar]
- Rutherford, A.W.; Renger, G.; Koike, H.; Inoue, Y. Thermoluminiscence as a probe of photosystem II. The redox and protonation states of the secondary acceptor quinone and O2-evolving enzyme. Biochim. Biophys. Acta-Bioenerg. 1984, 767, 548–556. [Google Scholar] [CrossRef]
- Chu, H.-A.; Nguyen, A.P.; Debus, R.J. Site-directed photosystem II mutants with perturbed oxygen-evolving properties. 1. Instability or inefficient assembly of the manganese cluster in vivo. Biochemistry 1994, 33, 6137–6149. [Google Scholar] [CrossRef]
- Hung, C.-H.; Huang, J.-Y.; Chiu, Y.-F.; Chu, H.-A. Site-directed mutagenesis on the heme axial-ligands of cytochrome b559 in photosystem II by using cyanobacteria Synechocystis PCC 6803. Biochim. Biophys. Acta-Bioenerg. 2007, 1767, 686–693. [Google Scholar] [CrossRef] [Green Version]
- Marchetti, A.; Schruth, D.M.; Durkin, C.A.; Parker, M.S.; Kodner, R.B.; Berthiaume, C.T.; Morales, R.; Allen, A.E.; Armbrust, E.V. Comparative metatranscriptomics identifies molecular bases for the physiological responses of phytoplankton to varying iron availability. Proc. Natl. Acad. Sci. USA 2012, 109, E317–E325. [Google Scholar] [CrossRef] [Green Version]
- Greene, R.M.; Geider, R.J.; Kolber, Z.; Falkowski, P.G. Iron-induced changes in light harvesting and photochemical energy conversion processes in eukaryotic marine algae. Plant Physiol. 1992, 100, 565–575. [Google Scholar] [CrossRef] [Green Version]
- Aro, E.-M.; Virgin, I.; Andersson, B. Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim. Biophys. Acta 1993, 1143, 113–134. [Google Scholar] [CrossRef]
- Oliver, N.; Avramov, A.P.; Nürnberg, D.J.; Dau, H.; Burnap, R.L. From manganese oxidation to water oxidation: Assembly and evolution of the water-splitting complex in photosystem II. Photosynth. Res. 2022, 152, 107–133. [Google Scholar] [CrossRef]
- McLachlan, J. Some considerations of the growth of marine algae in artificial media. Can. J. Microbiol. 1964, 10, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.C.; McCarthy, J.J. Steady state growth and ammonium uptake of a fast-growing marine diatom. Limnol. Oceanogr. 1978, 23, 695–703. [Google Scholar] [CrossRef]
- McKay, R.M.L.; La Roche, J.; Yakunin, A.F.; Drunford, D.G.; Geider, R.J. Accumulation of ferredoxin and flavodoxin in a marine diatom in response to Fe. J. Phycol. 1999, 35, 510–519. [Google Scholar] [CrossRef]
- Li, F.; Beardall, J.; Collins, S.; Gao, K. Decreased photosynthesis and growth with reduced respiration in the model diatom Phaeodactylum tricornutum grown under elevated CO2 over 1800 generations. Glob. Change Biol. 2017, 23, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Markwell, M.A.K.; Haas, S.M.; Bieber, L.L.; Tolbert, N.E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 1978, 87, 206–210. [Google Scholar] [CrossRef]
- Navarro, J.A.; Hervás, M.; de la Cerda, B.; de la Rosa, M.A. Purification and physiochemical properties of the low potential cytochrome c549 from the cyanobacterium Synechocystis sp. PCC 6803. Arch. Biochem. Biophys. 1995, 318, 46–52. [Google Scholar] [CrossRef]
- Bernal-Bayard, P.; Pallara, P.; Castell, C.; Molina-Heredia, F.P.; Fernández-Recio, J.; Hervás, M.; Navarro, J.A. Interaction of photosystem I from Phaeodactylum tricornutum with plastocyanins as compared with its native cytochrome c6: Reunion with a lost donor. Biochim. Biophys. Acta-Bioenerg. 2015, 1847, 1549–1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Calderón, M.; Betti, M.; Márquez, A.J.; Ortega, J.M.; Roncel, M. The afterglow thermoluminescence band as an indicator of changes in the photorespiratory metabolism of the model legume Lotus japonicus. Physiol. Plant. 2019, 166, 240–250. [Google Scholar] [CrossRef] [Green Version]
- Ducruet, J.M.; Miranda, T. Graphical and numerical analysis of thermoluminescence and fluorescence F0 emission in photosynthetic material. Photosynth. Res. 1992, 33, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Zurita, J.L.; Roncel, M.; Aguilar, M.; Ortega, J.M. A thermoluminescence study of photosystem II back electron transfer reactions in rice leaves. Effects of salt stress. Photosynth. Res. 2005, 84, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Ducruet, J.M.; Serrano, A.; Roncel, M.; Ortega, J.M. Peculiar properties of chlorophyll thermoluminescence emission of autotrophically or mixotrophically grown Chlamydomonas reinhardtii. J. Photochem. Photobiol. B Biol. 2011, 104, 301–307. [Google Scholar] [CrossRef] [PubMed]



| Parameter (after 15 Days of Growth) | 10 µM Fe | 0.1 µM Fe | 0.04 µM Fe |
|---|---|---|---|
| Specific growth rate (µ, day−1) | 0.252 ± 0.002 (100%) | 0.204 ± 0.009 (80.9%) | 0.190 ± 0.011 (75.4%) |
| Net respiration rate per 106 cells (nmol O2 h−1) | −0.57 ± 0.02 (100%) | −0.93 ± 0.01 (163.1%) | −0.82 ± 0.01 (142.6%) |
| Net photosynthetic rate per 106 cells (nmol O2 h−1) | 6.51 ± 0.03 (100%) | 3.16 ± 0.05 (48.5%) | 2.72 ± 0.04 (41.5%) |
| TL intensity (a.u.) | 340.9 ± 20.5 (100%) | 206.7 ± 17.3 (60.6%) | 161.2 ± 9.4 (47.3%) |
| Relative PSII content (Fm − F0) | 0.142 ± 0.037 (100%) | 0.084 ± 0.017 (59.1%) | 0.079 ± 0.021 (55.4%) |
| Maximum quantum yield of PSII (Fv/Fm) | 0.621 ± 0.016 (100%) | 0.463 ± 0.050 (74.5%) | 0.394 ± 0.014 (63.4%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castell, C.; Díaz-Santos, E.; Heredia-Martínez, L.G.; López-Maury, L.; Ortega, J.M.; Navarro, J.A.; Roncel, M.; Hervás, M. Iron Deficiency Promotes the Lack of Photosynthetic Cytochrome c550 and Affects the Binding of the Luminal Extrinsic Subunits to Photosystem II in the Diatom Phaeodactylum tricornutum. Int. J. Mol. Sci. 2022, 23, 12138. https://doi.org/10.3390/ijms232012138
Castell C, Díaz-Santos E, Heredia-Martínez LG, López-Maury L, Ortega JM, Navarro JA, Roncel M, Hervás M. Iron Deficiency Promotes the Lack of Photosynthetic Cytochrome c550 and Affects the Binding of the Luminal Extrinsic Subunits to Photosystem II in the Diatom Phaeodactylum tricornutum. International Journal of Molecular Sciences. 2022; 23(20):12138. https://doi.org/10.3390/ijms232012138
Chicago/Turabian StyleCastell, Carmen, Encarnación Díaz-Santos, Luis G. Heredia-Martínez, Luis López-Maury, José M. Ortega, José A. Navarro, Mercedes Roncel, and Manuel Hervás. 2022. "Iron Deficiency Promotes the Lack of Photosynthetic Cytochrome c550 and Affects the Binding of the Luminal Extrinsic Subunits to Photosystem II in the Diatom Phaeodactylum tricornutum" International Journal of Molecular Sciences 23, no. 20: 12138. https://doi.org/10.3390/ijms232012138