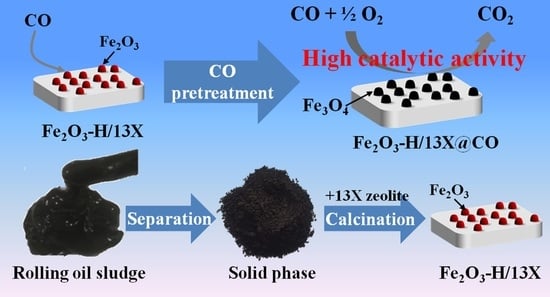
Solid Fe Resources Separated from Rolling Oil Sludge for CO Oxidation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physical Properties and Morphology of Fe Based Catalysts
2.2. Redox and Surface Properties
2.3. CO Oxidation Application
2.3.1. Effect of Calcination Temperatures, Different Synthesis Methods, and Pretreatment Conditions
2.3.2. CO Oxidation Activity on 13X Zeolite-Supported Catalysts
3. Methods and Materials
3.1. Materials and Reagents
3.2. Catalytic Hydrogenation Reaction
3.3. Catalyst Preparation
3.4. Catalyst Characterization
3.5. CO Oxidation Performance Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, S.; Li, T.; Wu, Y.; Chen, Y.; Su, T.; Ri, K.; Huo, Y. Effective purification of cold-rolling sludge as iron concentrate powder via a coupled hydrothermal and calcination route: From laboratory-scale to pilot-scale. J. Clean. Prod. 2020, 276, 124274. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, S.-g.; Steenari, B.-M.; Ekberg, C. Synthesis and properties of SrFe12O19 obtained by solid waste recycling of oily cold rolling mill sludge. Int. J. Miner. Metall. Mater. 2019, 26, 642–648. [Google Scholar] [CrossRef]
- Liang, Q.; Han, D.; Cao, Z.; Du, J. Studies on kinetic and reaction mechanism of oil rolling sludge under a wide temperature range. Energy Sources Part A 2021, 1–13. [Google Scholar] [CrossRef]
- Qin, L.; Han, J.; He, X.; Zhan, Y.; Yu, F. Recovery of energy and iron from oily sludge pyrolysis in a fluidized bed reactor. J. Environ. Manag. 2015, 154, 177–182. [Google Scholar] [CrossRef]
- Williams, D. Turning waste oil into profit. J. Can. Pet. Technol. 2009, 48, 7–8. [Google Scholar]
- Wang, Z.; Gong, Z.; Wang, Z.; Li, X.; Chu, Z. Application and development of pyrolysis technology in petroleum oily sludge treatment. Environ. Eng. Res. 2020, 26, 190460. [Google Scholar] [CrossRef]
- Chen, H.S.; Zhang, Q.M.; Yang, Z.J.; Liu, Y.S. Research on Treatment of Oily Sludge from the Tank Bottom by Ball Milling Combined with Ozone-Catalyzed Oxidation. ACS Omega 2020, 5, 12259–12269. [Google Scholar] [CrossRef]
- Chang, W.; Zhao, D.; Lian, J.; Han, S.; Xue, Y. Regeneration of waste rolling oil via inorganic flocculation-adsorption process. Pet. Sci. Technol. 2019, 37, 837–844. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, Z.; Xue, Y.; Chang, W.; Liu, P.; Han, S.; Lin, H. Regeneration of used rolling oil for sustainable use. Pet. Sci. Technol. 2017, 35, 1642–1647. [Google Scholar] [CrossRef]
- Zhao, J.; Yuan, C.; Yao, X. A simple method to evaluate solvent for lubricating oil by solubility parameter. Pet. Sci. Technol. 2017, 35, 1445–1450. [Google Scholar] [CrossRef]
- Adeyemi, A.F.; Adebiyi, F.M.; Koya, O.A. Evaluation of physico-chemical properties of re-refined lubricating oils obtained from fabricated packed bed reactor. Pet. Sci. Technol. 2017, 35, 1712–1723. [Google Scholar] [CrossRef]
- Ahmad, I.; Khan, R.; Ishaq, M.; Khan, H.; Ismail, M.; Gul, K.; Ahmad, W. Production of Lighter Fuels from Spent Lubricating Oil via Pyrolysis over Barium-Substituted Spinel Ferrite. Energy Fuel 2016, 30, 4781–4789. [Google Scholar] [CrossRef]
- Martín, M.I.; López, F.A.; Torralba, J.M. Production of sponge iron powder by reduction of rolling mill scale. Ironmak. Steelmak. 2013, 39, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Zang, S.-G.; Tian, J.-J.; Pan, D.-A.; Zhu, H.-X. Strontium ferrite powders prepared from oily cold rolling mill sludge by solid-state reaction method. Rare Met. 2013, 32, 518–523. [Google Scholar] [CrossRef]
- Park, J.-W.; Ahn, J.-C.; Song, H.; Park, K.; Shin, H.; Ahn, J.-S. Reduction characteristics of oily hot rolling mill sludge by direct reduced iron method. Resour. Conserv. Recycl. 2002, 34, 129–140. [Google Scholar] [CrossRef]
- Hocking, M.B. Petroleum Refining. In Handbook of Chemical Technology and Pollution Control; Academic Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Pujadó, P. Petroleum Refining Processes. J. Pet. Sci. Eng. 2002, 45, 295–296. [Google Scholar] [CrossRef]
- Heck, R.M.; Farrauto, R.J.; Gulati, S.T. Catalytic Air Pollution Control: Commercial Technology, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Freund, H.J.; Meijer, G.; Scheffler, M.; Schlögl, R.; Wolf, M. CO oxidation as a prototypical reaction for heterogeneous processes. Angew. Chem. Int. Ed. 2011, 50, 10064–10094. [Google Scholar] [CrossRef]
- Liu, B.; Wu, H.; Li, S.; Xu, M.; Cao, Y.; Li, Y. Solid-state construction of CuOx/Cu1.5Mn1.5O4 nanocomposite with abundant surface CuOx species and oxygen vacancies to promote CO oxidation activity. Int. J. Mol. Sci. 2022, 23, 6856. [Google Scholar] [CrossRef]
- Yang, L.; Shan, S.; Loukrakpam, R.; Petkov, V.; Ren, Y.; Wanjala, B.N.; Engelhard, M.H.; Luo, J.; Yin, J.; Chen, Y. Role of support–nanoalloy interactions in the atomic-scale structural and chemical ordering for tuning catalytic sites. J. Am. Chem. Soc. 2012, 134, 15048–15060. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Okejiri, F.; Luo, J.; Zhao, J.; Zhang, P.; Liu, M.; Yang, S.; Zhang, Z.; Song, W. Deep understanding of strong metal interface confinement: A journey of Pd/FeO x catalysts. ACS Catal. 2020, 10, 8950–8959. [Google Scholar] [CrossRef]
- Laguna, O.H.; Centeno, M.A.; Boutonnet, M.; Odriozola, J.A. Fe-doped ceria solids synthesized by the microemulsion method for CO oxidation reactions. Appl. Catal. B 2011, 106, 621–629. [Google Scholar] [CrossRef]
- Biabani-Ravandi, A.; Rezaei, M. Low temperature CO oxidation over Fe-Co mixed oxide nanocatalysts. Chem. Eng. J. 2012, 184, 141–146. [Google Scholar] [CrossRef]
- Papaioannou, E.I.; Neagu, D.; Ramli, W.K.W.; Irvine, J.T.S.; Metcalfe, I.S. Sulfur-Tolerant, Exsolved Fe-Ni Alloy Nanoparticles for CO Oxidation. Top. Catal. 2018, 62, 1149–1156. [Google Scholar] [CrossRef] [Green Version]
- Waqas, M.; Mountapmbeme Kouotou, P.; El Kasmi, A.; Wang, Y.; Tian, Z.-Y. Role of copper grid mesh in the catalytic oxidation of CO over one-step synthesized Cu-Fe-Co ternary oxides thin film. Chin. Chem. Lett. 2020, 31, 1201–1206. [Google Scholar] [CrossRef]
- Ramakrishna, C.; Krishna, R.; Gopi, T.; Swetha, G.; Saini, B.; Chandra Shekar, S.; Srivastava, A. Complete oxidation of 1,4-dioxane over zeolite-13X-supported Fe catalysts in the presence of air. Chin. J. Catal. 2016, 37, 240–249. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Guo, Y.; Zhu, T.; Xu, W. Adsorption and desorption characteristics of hydrophobic hierarchical zeolites for the removal of volatile organic compounds. Chem. Eng. J. 2021, 411, 128558. [Google Scholar] [CrossRef]
- Imran, M.; Affandi, A.M.; Alam, M.M.; Khan, A.; Khan, A.I. Advanced biomedical applications of iron oxide nanostructures based ferrofluids. Nanotechnology 2021, 32, 422001. [Google Scholar] [CrossRef]
- Imran, M.; Abutaleb, A.; Ashraf Ali, M.; Ahamad, T.; Rahman Ansari, A.; Shariq, M.; Lolla, D.; Khan, A. UV light enabled photocatalytic activity of α-Fe2O3 nanoparticles synthesized via phase transformation. Mater. Lett. 2020, 258, 126748. [Google Scholar] [CrossRef]
- Giecko, G.; Borowiecki, T.; Gac, W.; Kruk, J. Fe2O3/Al2O3 catalysts for the N2O decomposition in the nitric acid industry. Catal. Today 2008, 137, 403–409. [Google Scholar] [CrossRef]
- Sierra-Pereira, C.A.; Urquieta-González, E.A. Reduction of NO with CO on CuO or Fe2O3 catalysts supported on TiO2 in the presence of O2, SO2 and water steam. Fuel 2014, 118, 137–147. [Google Scholar] [CrossRef] [Green Version]
- Basińska, A.; Jóźwiak, W.K.; Góralski, J.; Domka, F. The behaviour of Ru/Fe2O3 catalysts and Fe2O3 supports in the TPR and TPO conditions. Appl. Catal. A 2000, 190, 107–115. [Google Scholar] [CrossRef]
- Tanaka, S.-I.; Yuzaki, K.; Ito, S.-I.; Kameoka, S.; Kunimori, K. Mechanism of O2 Desorption during N2O Decomposition on an Oxidized Rh/USY Catalyst. J. Catal. 2001, 200, 203–208. [Google Scholar] [CrossRef]
- Li, G.; Li, N.; Sun, Y.; Qu, Y.; Jiang, Z.; Zhao, Z.; Zhang, Z.; Cheng, J.; Hao, Z. Efficient defect engineering in Co-Mn binary oxides for low-temperature propane oxidation. Appl. Catal. B 2021, 282, 119512. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, Q.; Li, H.; Wang, J.; Tai, G.; Wang, F.; Han, J.; Zhu, Y.; Wu, G. Waste-to-resource strategy to fabricate functionalized MOFs composite material based on durian shell biomass carbon fiber and Fe3O4 for highly efficient and recyclable dye adsorption. Int. J. Mol. Sci. 2022, 23, 5900. [Google Scholar] [CrossRef]
- Kaur Ubhi, M.; Kaur, M.; Singh, D.; Javed, M.; Oliveira, A.C.; Kumar Garg, V.; Sharma, V.K. Hierarchical nanoflowers of MgFe2O4, bentonite and B-,P- Co-doped graphene oxide as adsorbent and photocatalyst: Optimization of parameters by Box-Behnken methodology. Int. J. Mol. Sci. 2022, 23, 9678. [Google Scholar] [CrossRef]
- Shekar, S.G.C.; Alkanad, K.; Hezam, A.; Alsalme, A.; Al-Zaqri, N.; Lokanath, N.K. Enhanced photo-Fenton activity over a sunlight-driven ignition synthesized α-Fe2O3-Fe3O4/CeO2 heterojunction catalyst enriched with oxygen vacancies. J. Mol. Liq. 2021, 335, 116186. [Google Scholar] [CrossRef]
- Tepluchin, M.; Casapu, M.; Boubnov, A.; Lichtenberg, H.; Wang, D.; Kureti, S.; Grunwaldt, J.-D. Fe and Mn-Based Catalysts Supported on γ-Al2O3 for CO Oxidation under O2-Rich Conditions. ChemCatChem 2014, 6, 1763–1773. [Google Scholar] [CrossRef]
- Schoch, R.; Bauer, M. Pollution Control Meets Sustainability: Structure-Activity Studies on New Iron Oxide-Based CO Oxidation Catalysts. ChemSusChem 2016, 9, 1996–2004. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Chang, Z.; Sun, X.; Li, Y. Crystal plane effect of Fe2O3 with various morphologies on CO catalytic oxidation. Catal. Commun. 2011, 12, 530–534. [Google Scholar] [CrossRef]
- Gao, Q.-X.; Wang, X.-F.; Di, J.-L.; Wu, X.-C.; Tao, Y.-R. Enhanced catalytic activity of α-Fe2O3 nanorods enclosed with {110} and {001} planes for methane combustion and CO oxidation. Catal. Sci. Technol. 2011, 1, 574–577. [Google Scholar] [CrossRef]
- Tang, X.; Wang, J.; Li, J.; Zhang, X.; La, P.; Jiang, X.; Liu, B. In-situ growth of large-area monolithic Fe2O3/TiO2 catalysts on flexible Ti mesh for CO oxidation. J. Mater. Sci. Technol. 2021, 69, 119–128. [Google Scholar] [CrossRef]
- Schlicher, S.; Prinz, N.; Bürger, J.; Omlor, A.; Singer, C.; Zobel, M.; Schoch, R.; Lindner, J.K.N.; Schünemann, V.; Kureti, S.; et al. Quality or Quantity? How Structural Parameters Affect Catalytic Activity of Iron Oxides for CO Oxidation. Catalysts 2022, 12, 675. [Google Scholar] [CrossRef]
- Li, P.; Miser, D.E.; Rabiei, S.; Yadav, R.T.; Hajaligol, M.R. The removal of carbon monoxide by iron oxide nanoparticles. Appl. Catal. B 2003, 43, 151–162. [Google Scholar] [CrossRef]
- Carriazo, J.G.; Centeno, M.A.; Odriozola, J.A.; Moreno, S.; Molina, R. Effect of Fe and Ce on Al-pillared bentonite and their performance in catalytic oxidation reactions. Appl. Catal. A 2007, 317, 120–128. [Google Scholar] [CrossRef]










| Sample | Peak Location (eV) | Fe2+/(Fe2+ + Fe3+) a | Oa/O b | Ob/O c | ||
|---|---|---|---|---|---|---|
| Fe2+oct | Fe3+oct | Fe3+tet | ||||
| Fe2O3-H | - | - | - | - | 66.4% | 33.6% |
| Fe2O3-C | - | - | - | - | 71.2% | 28.8% |
| 20% Fe2O3-H/13X | - | - | - | - | 18.0% | 82.0% |
| 20% Fe2O3-C/13X | - | - | - | - | 19.4% | 80.6% |
| Fe2O3-H@CO | 710.06 | 711.24 | 713.12 | 29.6% | 61.2% | 38.8% |
| Fe2O3-C@CO | 709.92 | 711.15 | 712.92 | 27.1% | 68.2% | 31.8% |
| 20% Fe2O3-H/13X@CO | 709.90 | 711.30 | 713.06 | 34.6% | 11.5% | 88.5% |
| 20% Fe2O3-C/13X@CO | 709.9 | 711.15 | 712.98 | 20.1% | 17.5% | 82.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, W.; Tang, S.; Wu, T.; Wu, J.; Cheng, K.; Xia, M. Solid Fe Resources Separated from Rolling Oil Sludge for CO Oxidation. Int. J. Mol. Sci. 2022, 23, 12134. https://doi.org/10.3390/ijms232012134
Gao W, Tang S, Wu T, Wu J, Cheng K, Xia M. Solid Fe Resources Separated from Rolling Oil Sludge for CO Oxidation. International Journal of Molecular Sciences. 2022; 23(20):12134. https://doi.org/10.3390/ijms232012134
Chicago/Turabian StyleGao, Wei, Sai Tang, Ting Wu, Jianhong Wu, Kai Cheng, and Minggui Xia. 2022. "Solid Fe Resources Separated from Rolling Oil Sludge for CO Oxidation" International Journal of Molecular Sciences 23, no. 20: 12134. https://doi.org/10.3390/ijms232012134