Structural Studies on Diverse Betacyanin Classes in Matured Pigment-Rich Fruits of Basella alba L. and Basella alba L. var. ‘Rubra’ (Malabar Spinach)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Non-Acylated Polar Betacyanins Identified in the Fruits of B. alba and var. ‘Rubra’
2.2. Malonylated Betanidin 6-O-β-Glusosides and Their Acyl Migration Derivatives
2.3. 3-Hydroxy-Butyrylated Betanidin 6-O-β-Glusosides
2.4. Hydroxycinnamic Acid Conjugates of Gomphrenin
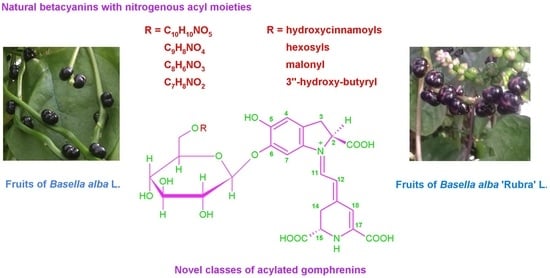
2.5. Novel Natural Betacyanins Acylated with Nitrogenous Substituents
2.6. NMR Structural Elucidation of Acylated Gomphrenins
2.7. Quantification of Betacyanins in the Fruits of B. alba and B. alba var. ‘Rubra’
3. Materials and Methods
3.1. Reagents
3.2. Plant Material
3.3. Betacyanin Reference Material
3.4. Preparation of Juice from B. alba and B. var. ‘Rubra’ Fruits
3.5. Fast Betacyanin Screening in the Fruit Juice Samples
3.6. Pigment Purification for LC-MS Experiments
3.7. Preparation of Isolated Betacyanins from the Purified B. alba Extract
3.8. Chromatographic Analysis with Detection by a Low-Resolution Mass Spectrometric System (LC-DAD-ESI-MS/MS)
3.9. Chromatographic Analysis with Detection by a High-Resolution Mass Spectrometric System (LC-Q-Orbitrap-MS)
3.10. NMR Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaurasiya, A.; Pal, R.K.; Verma, P.K.; Katiyar, A.; Razauddin; Narendra, K.J. An updated review on Malabar spinach (Basella alba and Basella rubra) and their importance. J. Pharmacogn. Phytochem. 2021, 10, 1201–1207. [Google Scholar] [CrossRef]
- Adhikari, R.; Kumar, N.H.; Shruthi, S.D. A review on medicinal importance of Basella alba L. Int. J. Pharm. Sci. Drug Res. 2012, 4, 110–114. [Google Scholar]
- Deka, J.; Borah, U.; Dash, B.; Dash, S.; Kalita, L. Preliminary phytochemical screening and in vitro antimicrobial activity of ethanolic extract of stem of the herb Basella alba L. var Rubra (L.) Stewart (Family-Basellaceae). Int. J. Curr. Pharm. Res. 2017, 9, 91–94. [Google Scholar] [CrossRef]
- Kumar, S.S.; Manoj, P.; Giridhar, P. Nutrition facts and functional attributes of foliage of Basella spp. LWT-Food Sci. Technol. 2015, 64, 468–474. [Google Scholar] [CrossRef]
- Kumar, S.S.; Manoj, P.; Giridhar, P.; Shrivastava, R.; Bharadwaj, M. Fruit extracts of Basella rubra that are rich in bioactives and betalains exhibit antioxidant activity and cytotoxicity against human cervical carcinoma cells. J. Funct. Foods 2015, 15, 509–515. [Google Scholar] [CrossRef]
- Kumar, S.S.; Manoj, P.; Nimisha, G.; Giridhar, P. Phytoconstituents and stability of betalains in fruit extracts of Malabar spinach (Basella rubra L.). J. Food Sci. Technol. 2016, 53, 4014–4022. [Google Scholar] [CrossRef]
- Kumorkiewicz-Jamro, A.; Świergosz, T.; Sutor, K.; Spórna-Kucab, A.; Wybraniec, S. Multi-colored shades of betalains: Recent advances in betacyanin chemistry. Nat. Prod. Rep. 2021, 38, 2315–2346. [Google Scholar] [CrossRef]
- Kumorkiewicz, A.; Szneler, E.; Wybraniec, S. Conjugation of oxidized betanidin and gomphrenin pigments from Basella alba L. fruits with glutathione. J. Agric. Food Chem. 2018, 66, 12815–12826. [Google Scholar] [CrossRef]
- Mabry, T.; Dreiding, A.S. The betalains. In Recent Advances in Phytochemistry; Mabry, T.J., Alston, R.E., Runeckles, V.C., Eds.; Appleton-Century-Crofts: New York, NY, USA, 1968; Volume 1, pp. 145–160. [Google Scholar]
- Mabry, T.J. Selected topics from forty years of natural products research: Betalains to flavonoids, antiviral proteins, and neurotoxic nonprotein amino acids. J. Nat. Prod. 2001, 64, 1596–1604. [Google Scholar] [CrossRef]
- Zrÿd, J.P.; Christinet, L. Betalains. In Plant Pigments and Their Manipulation. Annual Plant Reviews; Davies, K., Ed.; Wiley-Blackwell: Chichester, UK, 2004; Volume 14, pp. 185–213. [Google Scholar]
- Bastos, E.L.; Schliemann, W. Betalains as Antioxidants. In Plant Antioxidants and Health; Ekiert, H.M., Ramawat, K.G., Arora, J., Eds.; Reference Series in Phytochemistry; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–44. [Google Scholar]
- Khan, M.I.; Giridhar, P. Plant betalains: Chemistry and biochemistry. Phytochemistry 2015, 117, 267–295. [Google Scholar] [CrossRef]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Betalain stability and degradation—Structural and chromatic aspects. J. Food Sci. 2006, 71, 41–50. [Google Scholar] [CrossRef]
- Spórna-Kucab, A.; Milo, A.; Kumorkiewicz, A.; Wybraniec, S. Studies on polar high-speed counter-current chromatographic systems in separation of amaranthine-type betacyanins from Celosia species. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1073, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Wybraniec, S.; Jerz, G.; Gebers, N.; Winterhalter, P. Ion-pair high-speed countercurrent chromatography in fractionation of a high-molecular weight variation of acyl-oligosaccharide linked betacyanins from purple bracts of Bougainvillea glabra. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 538–550. [Google Scholar] [CrossRef]
- Sravan Kumar, S.; Manoj, P.; Giridhar, P. A method for red-violet pigments extraction from fruits of Malabar spinach (Basella rubra) with enhanced antioxidant potential under fermentation. J. Food Sci. Technol. 2015, 52, 3037–3043. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. Biological activities of plant pigments betalains. Crit. Rev. Food Sci. Nutr. 2016, 56, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I. Plant betalains: Safety, antioxidant activity, clinical efficacy and bioavailability. Compr. Rev. Food Sci. Food Saf. 2016, 15, 316–330. [Google Scholar] [CrossRef]
- Esquivel, P. Betalains. In Handbook on Natural Pigments in Food and Beverages: Industrial Applications for Improving Food Color; Carle, R., Schweiggert, R., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 81–99. [Google Scholar]
- Castellar, R.; Obón, J.M.; Alacid, M.; Fernández-López, J.A. Color properties and stability of betacyanins from Opuntia fruits. J. Agric. Food Chem. 2003, 51, 2772–2776. [Google Scholar] [CrossRef]
- Schliemann, W.; Strack, D. Intramolecular stabilization of acylated betacyanins. Phytochemistry 1998, 49, 585–588. [Google Scholar] [CrossRef]
- Kumar, S.S.; Arya, M.; Chauhan, A.S.; Giridhar, P. Basella rubra fruit juice betalains as a colorant in food model systems and shelf-life studies to determine their realistic usability. J. Food Process. Preserv. 2020, 44, e14595. [Google Scholar] [CrossRef]
- Kumar, S.S.; Manoj, P.; Shetty, N.P.; Prakash, M.; Giridhar, P. Characterization of major betalain pigments—Gomphrenin, betanin and isobetanin from Basella rubra L. fruit and evaluation of efficacy as a natural colourant in product (ice cream) development. J. Food Sci. Technol. 2015, 52, 4994–5002. [Google Scholar] [CrossRef]
- Kumar, S.S.; Chauhan, A.S.; Giridhar, P. Nanoliposomal encapsulation mediated enhancement of betalain stability: Characterisation, storage stability and antioxidant activity of Basella rubra L. fruits for its applications in vegan gummy candies. Food Chem. 2020, 333, 127442. [Google Scholar] [CrossRef] [PubMed]
- Wybraniec, S.; Nowak-Wydra, B.; Mitka, K.; Kowalski, P.; Mizrahi, Y. Minor betalains in fruits of Hylocereus species. Phytochemistry 2007, 68, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, T.; Bączek, N.; Wiczkowski, W. Betalain profile, content and antioxidant capacity of red beetroot dependent on the genotype and root part. J. Funct. Foods 2016, 27, 249–261. [Google Scholar] [CrossRef]
- Sutor, K.; Wybraniec, S. Identification and determination of betacyanins in fruit extracts of Melocactus species. J. Agric. Food Chem. 2020, 68, 11459–11467. [Google Scholar] [CrossRef] [PubMed]
- Glassgen, W.E.; Metzger, J.W.; Heuer, S.; Strack, D. Betacyanins from fruits of Basella rubra. Phytochemistry 1993, 33, 1525–1527. [Google Scholar] [CrossRef]
- Schwartz, S.J.; von Elbe, J.H. Quantitative determination of individual betacyanin pigments by high-performance liquid chromatography. J. Agric. Food Chem. 1980, 28, 540–543. [Google Scholar] [CrossRef]
- Heuer, S.; Wray, V.; Metzger, J.W.; Strack, D. Betacyanins from flowers of Gomphrena globosa. Phytochemistry 1992, 31, 1801–1807. [Google Scholar] [CrossRef]
- Imperato, F. Acylated betacyanins of Portulaca oleracea. Phytochemistry 1975, 14, 2091–2092. [Google Scholar] [CrossRef]
- Wybraniec, S. Chromatographic investigation on acyl migration in betacyanins and their decarboxylated derivatives. J. Chromatogr. B Biomed. Sci. Appl. 2008, 861, 40–47. [Google Scholar] [CrossRef]
- Kugler, F.; Stintzing, F.C.; Carle, R. Characterisation of betalain patterns of differently coloured inflorescences from Gomphrena globosa L. and Bougainvillea sp. by HPLC-DAD-ESI-MSn. Anal. Bioanal. Chem. 2006, 387, 637–648. [Google Scholar] [CrossRef]
- Lystvan, K.; Kumorkiewicz, A.; Szneler, E.; Wybraniec, S. Study on betalains in Celosia cristata Linn. callus culture and identification of new malonylated amaranthins. J. Agric. Food Chem. 2018, 66, 3870–3879. [Google Scholar] [CrossRef] [PubMed]
- Stintzing, F.C.; Schieber, A.; Carle, R. Betacyanins in fruits from red-purple pitaya, Hylocereus polyrhizus (Weber) Britton & Rose. Food Chem. 2002, 77, 101–106. [Google Scholar]
- Stintzing, F.C.; Conrad, J.; Klaiber, I.; Beifuss, U.; Carle, R. Structural investigations on betacyanin pigments by LC NMR and 2D NMR spectroscopy. Phytochemistry 2004, 65, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Wybraniec, S.; Nowak-Wydra, B.; Mizrahi, Y. 1H and 13C NMR spectroscopic structural elucidation of new decarboxylated betacyanins. Tetrahedron Lett. 2006, 47, 1725–1728. [Google Scholar] [CrossRef]
- Wybraniec, S.; Nowak-Wydra, B. Mammillarinin—A new malonylated betacyanin in fruits of Mammillaria. J. Agric. Food Chem. 2007, 55, 8138–8143. [Google Scholar] [CrossRef]
- Strack, D.; Steglich, W.; Wray, V. Betalains. In Methods in Plant Biochemistry; Dey, P.M., Harborne, J.B., Waterman, P.G., Eds.; Academic Press: London, UK, 1993; Volume 8, pp. 421–450. [Google Scholar]
- Stintzing, F.C.; Kugler, F.; Carle, R.; Conrad, J. First 13C-NMR Assignments of betaxanthins. Helv. Chim. Acta 2006, 89, 1008–1016. [Google Scholar] [CrossRef]
- Lin, S.M.; Lin, B.H.; Hsieh, W.M.; Ho, H.J.; Liu, C.D.; Chen, L.G.; Chiou, R.Y. Structural identification and bioactivities of red-violet pigments present in Basella alba fruits. J. Agric. Food Chem. 2010, 58, 10364–10372. [Google Scholar] [CrossRef]




| No. | Compound | Rt (min) | λmax (nm) | m/z [M+H]+ | m/z LC-ESI-(+)-MS/MS |
|---|---|---|---|---|---|
| 1 | betanidin 5-O-β-sophoroside (melocactin) | 5.5 | 537 | 713 | 551; 389 |
| 2 | (hexosyl)-(hexosyl)-betanidin a | 5.7 | - b | 713 | 551; 389 |
| 3 | (hexosyl)-betanidin a | 5.7 | 539 | 551 | 389 |
| 1’ | isobetanidin 5-O-β-sophoroside (isomelocactin) | 5.9 | 537 | 713 | 551; 389 |
| 3’ | (hexosyl)-isobetanidin a | 6.2 | 539 | 551 | 389 |
| 2’ | (hexosyl)-(hexosyl)-isobetanidin a | 6.3 | 537 | 713 | 551; 389 |
| 4 | betanidin 5-O-β-glucoside (betanin) | 6.6 | 535 | 551 | 389 |
| 5 | betanidin 6-O-β-sophoroside (bougainvillein-v) | 6.8 | 542 | 713 | 551; 389 |
| 6 | (hexosyl)-(hexosyl)-betanidin a | 6.9 | 536 | 713 | 551; 389 |
| 4’ | Isobetanidin 5-O-β-glucoside (isobetanin) | 7.3 | 535 | 551 | 389 |
| 5’ | isobetanidin 6-O-β-sophoroside (isobougainvillein-v) | 7.3 | 542 | 713 | 551; 389 |
| 6’ | (hexosyl)-(hexosyl)-isobetanidin a | 7.5 | 536 | 713 | 551; 389 |
| 7 | betanidin 6-O-β-glucoside (gomphrenin) | 8.2 | 537 | 551 | 389 |
| 7’ | isobetanidin 6-O-β-glucoside (isogomphrenin) | 8.7 | 537 | 551 | 389 |
| 8a | 3’-O-malonyl-gomphrenin a | 8.7 | 536 | 637 | 593; 551; 389 |
| 8b | 4’-O-malonyl-gomphrenin a | 9.1 | 538 | 637 | 593; 551; 389 |
| 8a’ | 3’-O-malonyl-isogomphrenin a | 9.5 | 538 | 637 | 593; 551; 389 |
| 8c | 6’-O-malonyl-gomphrenin a | 9.7 | 537 | 637 | 593; 551; 389 |
| 8b’ | 4’-O-malonyl-isogomphrenin a | 10.0 | 538 | 637 | 593; 551; 389 |
| 8c’ | 6’-O-malonyl-isogomphrenin a | 10.3 | 537 | 637 | 593; 551; 389 |
| 9 | C9H8NO4-gomphrenin a | 10.4 | 543 | 744 | 700; 656; 612; 568; 551; 389 |
| 10a | (3’’-hydroxy-butyryl)-(hexosyl)-betanidin a | 10.7 | 537 | 637 | 551; 389 |
| 11 | C10H10NO5-gomphrenin a | 10.8 | 542 | 774 | 742; 389 |
| 12 | (hexosyl)-(coumaroyl-hexosyl)-betanidin a | 11.0 | 543 | 859 | 697; 551; 389 |
| 10b | (3’’-hydroxy-butyryl)-(hexosyl)-isobetanidin a | 11.0 | 537 | 637 | 551; 389 |
| 9’ | C9H8NO4-isogomphrenin a | 11.2 | 543 | 744 | 700; 656; 612; 568; 551; 389 |
| 10c | 3’’-hydroxy-butyryl-gomphrenin a | 11.4 | 538 | 637 | 551; 389 |
| 12’ | (hexosyl)-(coumaroyl-hexosyl)-isobetanidin a | 11.5 | 543 | 859 | 697; 551; 389 |
| 11’ | C10H10NO5-isogomphrenin a | 11.8 | - b | 774 | - |
| 10d | 3’’-hydroxy-butyryl-isogomphrenin a | 11.9 | 538 | 637 | 551; 389 |
| 13 | C8H6NO3-gomphrenin a | 12.6 | 542 | 714 | 670; 626; 582; 551; 538; 389 |
| 14 | C7H8NO2-gomphrenin a | 12.7 | 538 | 688 | 644; 600; 389 |
| 13’ | C8H6NO3-isogomphrenin a | 12.9 | 542 | 714 | 670; 626; 582; 551; 538; 389 |
| 15 | 6’O-E-caffeoyl-gomphrenin (malabarin) | 12.9 | 545 | 713 | 551; 389 |
| 14’ | C7H8NO2-isogomphrenin a | 13.1 | 538 | 688 | 644; 600; 389 |
| 16 | Z-isomer of globosin a | 13.4 | 544 | 697 | 653; 551; 389 |
| 17 | Z-isomer of basellin a | 13.4 | 544 | 727 | 551; 389 |
| 15’ | 6’O-E-caffeoyl-isogomphrenin (isomalabarin) | 13.6 | 545 | 713 | 551; 389 |
| 18 | (hexosyl)-(coumaroyl-hexosyl)-betanidin a | 13.7 | 543 | 859 | 697; 551; 389 |
| 16’ | Z-isomer of isoglobosin a | 13.8 | 544 | 697 | 653; 551; 389 |
| 17’ | Z-isomer of isobasellin a | 13.8 | 545 | 727 | 551; 389 |
| 18’ | (hexosyl)-(coumaroyl-hexosyl)-isobetanidin a | 13.9 | 543 | 859 | 697; 551; 389 |
| 19 | 6’O-E-sinapoyl-gomphrenin (gandolin) | 14.3 | 544 | 757 | 713; 551; 389 |
| 20 | 6’O-E-4-coumaroyl-gomphrenin (globosin) | 14.5 | 544 | 697 | 653; 551; 389 |
| 21 | 6’O-E-feruloyl-gomphrenin (basellin) | 14.5 | 545 | 727 | 551; 389 |
| 20’ | 6’O-E-4-coumaroyl-isogomphrenin (isoglobosin) | 15.3 | 544 | 697 | 653; 551; 389 |
| 19’ | 6’O-E-sinapoyl-isogomphrenin (isogandolin) | 15.5 | 544 | 757 | 551; 389 |
| 21’ | 6’O-E-feruloyl-isogomphrenin (isobasellin) | 15.5 | 545 | 727 | 551; 389 |
| No. | Compound a | Molecular Formula | [M+H]+ Observed | [M+H]+ Predicted | Error (mDa) | Error (ppm) | MS2 Ions |
|---|---|---|---|---|---|---|---|
| 2 | hex-hex-Bd | C30H37N2O18 | 713.2030 | 713.2036 | −0.6 | −0.84 | 551.1502 (-hex); 389.0975 (-hex-hex) |
| 3 | hex-Bd | C24H27N2O13 | 551.1505 | 551.1508 | −0.3 | −0.54 | 389.0973 (-hex) |
| 6 | hex-hex-Bd | C30H37N2O18 | 713.2033 | 713.2036 | −0.3 | −0.42 | 695.1904 (-H2O); 551.1502 (-hex); 389.0975 (-hex-hex) |
| 8a | 3’-mal-Gp | C27H29N2O16 | 637.1507 | 637.1512 | −0.5 | −0.78 | 619.1409 (-H2O); 593.1608 (-CO2); 551.1500 (-mal); 389.0976 (-mal-glc) |
| 8b | 4’-mal-Gp | C27H29N2O16 | 637.1508 | 637.1512 | −0.4 | −0.63 | 619.1413 (-H2O); 593.1611 (-CO2); 551.1503 (-mal); 389.0973 (-mal-glc) |
| 8c | 6’-mal-Gp | C27H29N2O16 | 637.1513 | 637.1512 | 0.1 | 0.16 | 619.1402 (-H2O); 593.1603 (-CO2); 551.1497 (-mal); 389.0977 (-mal-glc) |
| 10a | (3’’-OH-but)-hex-Bd | C28H33N2O15 | 637.1870 | 637.1876 | −0.6 | −0.94 | 593.1978 (-CO2); 551.1498 (-3-OH-but); 389.0968 (-3-OH-but-glc) |
| 10b | (3’’-OH-but)-hex-Bd | C28H33N2O15 | 637.1869 | 637.1876 | −0.7 | −1.10 | 593.1973 (-CO2); 551.1492 (-3-OH-but); 389.0977 (-3-OH-but-glc) |
| 10c | 3’’-OH-but-Gp | C28H33N2O15 | 637.1875 | 637.1876 | −0.1 | −0.16 | 593.1982 (-CO2); 551.1495 (-3-OH-but); 389.0971 (-3-OH-but-glc) |
| 10d | 3’’-OH-but-isoGp | C28H33N2O15 | 637.1871 | 637.1876 | −0.5 | −0.78 | 593.1976 (-CO2); 551.1497 (-3-OH-but); 389.0973 (-3-OH-but-glc) |
| 12 | hex-(coum)-hex-Gp | C39H43N2O20 | 859.2398 | 859.2404 | −0.6 | −0.70 | 815.2499 (-CO2); 697.1868 (-hex); 653.1984 (-hex, -CO2); 551.1502 (-coum-hex); 389.0979 (-coum-hex-hex) |
| 15 | caff-Gp | C33H33N2O16 | 713.1820 | 713.1825 | −0.5 | −0.70 | 669.1913 (-CO2); 625.2020 (-2CO2); 551.1503 (-caff); 389.0973 (-caff-glc) |
| 16 | Z-coum-Gp | C33H33N2O15 | 697.1874 | 697.1876 | −0.2 | −0.29 | 653.1985 (-CO2); 609.2075 (-2CO2); 551.1499 (-Z-coum); 389.0973 (-Z-coum-glc) |
| 17 | Z-fer-Gp | C34H35N2O16 | 727.1982 | 727.1981 | 0.1 | 0.14 | 683.2061 (-CO2); 551.1495 (-Z-fer); 389.0971 (-Z-fer-glc) |
| 18 | hex-(coum)-hex-Gp | C39H43N2O20 | 859.2407 | 859.2404 | 0.3 | 0.35 | 841.2291 (-H2O); 713.2050 (-coum); 697.1877 (-hex); 551.1503 (-coum-hex); 389.0972 (-coum-hex-hex) |
| 19 | sin-Gp | C35H37N2O17 | 757.2083 | 757.2087 | −0.4 | −0.53 | 713.2178 (-CO2); 669.2291 (-2CO2); 551.1502 (-sin); 389.0973(-sin-glc) |
| No. | Compound a | Molecular Formula | [M+H]+ Observed | [M+H]+ Predicted | Error (mDa) | Error (ppm) |
|---|---|---|---|---|---|---|
| 9 | [C9H8NO4-Gp +H]+ | C33H34N3O17 | 744.1878 | 744.1883 | −0.5 | −0.67 |
| nl: -CO2 | C32H34N3O15 | 700.1988 | 700.1984 | 0.4 | 0.51 | |
| nl: -2CO2 | C31H34N3O13 | 656.2078 | 656.2086 | −0.8 | −1.24 | |
| nl: -3CO2 | C30H34N3O11 | 612.2181 | 612.2188 | −0.7 | −1.12 | |
| nl: -4CO2 | C29H34N3O9 | 568.2274 | 568.2290 | −1.6 | −2.74 | |
| nl: -C9H8NO4 | C24H27N2O13 | 551.1529 | 551.1508 | 2.1 | 3.81 | |
| nl: -C9H8NO4-glc | C18H17N2O8 | 389.0973 | 389.0979 | −0.6 | −1.65 | |
| 11 | [C10H10NO5-Gp +H]+ | C34H36N3O18 | 774.1975 | 774.1988 | −1.3 | −1.68 |
| nl: -CH3OH | C33H32N3O17 | 742.1715 | 742.1726 | −1.1 | −1.48 | |
| nl: -CH3OH; -CO2 | C32H32N3O15 | 698.1834 | 698.1828 | 0.6 | 0.86 | |
| nl: -CH3OH; -2CO2 | C31H32N3O13 | 654.1935 | 654.1930 | 0.5 | 0.76 | |
| nl: -CH3OH; -3CO2 | C30H32N3O11 | 610.2034 | 610.2031 | 0.3 | 0.49 | |
| nl: -CH3OH; -4CO2 | C29H32N3O9 | 566.2142 | 566.2133 | 0.9 | 1.59 | |
| nl: -C10H10NO5-glc | C18H17N2O8 | 389.0973 | 389.0979 | −0.6 | −1.54 | |
| 13 | [C8H6NO3-Gp +H]+ | C32H32N3O16 | 714.1776 | 714.1777 | −0.1 | −0.14 |
| nl: -CO2 | C31H32N3O14 | 670.1875 | 670.1879 | −0.4 | −0.57 | |
| nl: -2CO2 | C30H32N3O12 | 626.1965 | 626.1981 | −1.5 | −2.48 | |
| nl: -3CO2 | C29H32N3O10 | 582.2072 | 582.2082 | −1.0 | −1.75 | |
| nl: -C8H6NO3 | C24H27N2O13 | 551.1495 | 551.1508 | −1.3 | −2.36 | |
| nl: -4CO2 | C28H32N3O8 | 538.2166 | 538.2184 | −1.8 | −3.33 | |
| nl: -C8H6NO3-glc | C18H17N2O8 | 389.0974 | 389.0979 | −0.5 | −1.39 | |
| 14 | [C7H8NO2-Gp +H]+ | C31H34N3O15 | 688.1983 | 688.1984 | −0.1 | −0.15 |
| nl: -CO2 | C30H34N3O13 | 644.2069 | 644.2086 | −1.7 | −2.66 | |
| nl: -2CO2 | C29H34N3O11 | 600.2170 | 600.2188 | −1.8 | −2.97 | |
| nl: -3CO2 | C28H34N3O9 | 556.2283 | 556.2290 | −0.7 | −1.18 | |
| nl: -C7H8NO2-glc | C18H17N2O8 | 389.0978 | 389.0979 | −0.1 | −0.36 |
| 6’-O-E-caffeoyl-gomphrenin 15 | 6’-O-E-sinapoyl-gomphrenin 19 | 6’-O-E-4-coumaroyl-gomphrenin 20 | 6’-O-E-feruloyl-gomphrenin 21 | |||||
|---|---|---|---|---|---|---|---|---|
| No. | 1H NMR a | 13C b,c | 1H NMR a | 13C b,c | 1H NMR a | 13C b,c | 1H NMR a | 13C b,c |
| 2 | 3.88, bm | 67.5 | 4.72, dd, 3.2; 10.0 | 64.5 | 3.85, bdd, | 65.2 | 3.79, dd, 3.3; 10.2 | 64.7 |
| 3a/b | 3.31, bm 3.04 (overlap) | 35.9 | 3.38, dd, 10.2; 16.3 3.26, dd, 16.8; 3.0 | 33.4 | 3.34, dd, 10.4; 16.6 3.13, dd, 16.3; 3.7 | 33.9 | 3.29, dd, 10.3; 16.1 3.12, dd, 16.6; 3.2 | 34.0 |
| 4 | 6.76, s | 116.2 | 6.87, s | 113.8 | 6.80 (overlap) | 114.1 | 6.79, s | 113.9 |
| 5 | 145.8 | 146.9 | 144.7 | 144.8 | ||||
| 6 | 146.8 | 149.6 | 145.7 | 145.8 | ||||
| 7 | 6.84, s | 100.9 | 7.42, s | 103.0 | 6.80, s | 98.9 | 6.77 (overlap) | 98.6 |
| 8 | 137.1 | 134.4 | 135.1 | 135.0 | ||||
| 9 | 129.3 | 129.1 | 127.2 | 126.7 | ||||
| 10 | 178.2 | 171.1 | 175.4 | 174.7 | ||||
| 11 | 7.88, d, 12.5 | 144.4 | 8.58, d, 12.3 | 147.0 | 8.05, d, 12.5 | 143.0 | 8.03, d, 12.4 | 143.3 |
| 12 | 5.27, d, 12.3 | 109.5 | 5.93, d, 12.2 | 110.5 | 5.38, d, 12.4 | 108.4 | 5.39, d, 12.3 | 108.4 |
| 13 | 164.4 | 163.0 | 162.4 | 162.0 | ||||
| 14a/b | 3.08 (overlap) 3.02 (overlap) | 29.9 | 3.67, dd, 17.5; 5.1 3.20, bs | 27.7 | 3.22, bm 3.04, bm | 27.9 | 3.20, bm 3.04, bm | 27.9 |
| 15 | 4.28, bm | 56.4 | 4.53, bt, 7.2 | 53.2 | 4.41, bt, 9.2 | 53.9 | 4.47, bt, 6.8 | 53.5 |
| 17 | 158.4 | 150.2 | 156.0 | 155.0 | ||||
| 18 | 6.04, bs | 107.5 | 6.33, bs | 106.6 | 6.13, bs | 105.6 | 6.11, s | 105.6 |
| 19 | 178.5 | 172.9 | 175.8 | 175.0 | ||||
| 20 | 170.4 | 167.4 | 169.3 | 167.8 | ||||
| 1’ | 5.01, d, 7.1 d | 100.6 | 4.96, d, 7.7 | 102.1 | 5.11, d, 6.7 d | 98.6 | 4.85, d, 6.9 d | 98.4 |
| 2’ | 3.64 (overlap) | 78.3 | 3.53 (overlap) | 77.4 | 3.61 (overlap) | 76.4 | 3.61(overlap) | 76.4 |
| 3’ | 3.63 (overlap) | 75.3 | 3.58 (overlap) | 74.4 | 3.66 (overlap) | 73.5 | 3.66 (overlap) | 73.3 |
| 4’ | 3.43 (overlap) | 73.8 | 3.45 (overlap) | 71.9 | 3.44 (overlap) | 71.8 | 3.44 (overlap) | 71.9 |
| 5’ | 3.96 (overlap) | 76.8 | 3.84 (overlap) | 75.2 | 3.96 (overlap) | 75.1 | 3.97 (overlap) | 74.6 |
| 6’a/b | 4.49, dd, 11.9; 4.9 4.37, dd, 12.1; 2.2 | 66.3 | 4.68, dd, 11.9; 5.3 4.42, dd, 11.8; 2.5 | 64.2 | 4.49 (overlap) 4.48 (overlap) | 64.5 | 4.54, dd, 12.0; 5.1 4.34, dd, 11.7; 2.1 | 64.4 |
| 1″ | 128.9 | 126.4 | 126.9 | 127.3 | ||||
| 2″ | 6.79, bd | 118.8 | 6.69, s | 106.9 | 7.09 (overlap) | 131.3 | 6.78 (overlap) | 111.3 |
| 3″ | 146.6 | 149.6 | 6.86 (overlap) | 116.7 | 148.5 | |||
| 4″ | 149.7 | 139.7 | 159.5 | 149.0 | ||||
| 5″ | 6.69, bd | 117.8 | 149.6 | 6.86 (overlap) | 116.7 | 6.86, d, 8.0 | 116.2 | |
| 6″ | 6.58, bdd | 125.4 | 6.69, s | 106.9 | 7.09 (overlap) | 131.3 | 6.57, bdd, 7.5 | 124.4 |
| 7″ | 7.01, d, 15.1 | 148.2 | 7.39, d, 16.4 | 147.3 | 7.11 (overlap) | 146.6 | 7.01, d, 16.4 | 146.6 |
| 8″ | 6.03, d, 15.6 | 117.0 | 6.47, d, 15.8 | 116.0 | 6.05, d, 15.9 | 114.8 | 6.08, d, 15.9 | 115.0 |
| 9″ | 171.4 | 169.3 | 169.7 | 169.4 | ||||
| 10″ | 3.81, s | 56.8 | 3.70, s | 56.7 | ||||
| 11″ | 3.81, s | 56.8 | ||||||
| No. | Relative Betacyanin Concentration (%) ± SD a | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound/ | B. alba | B. alba var. ‘Rubra’ | |||||||
| /Abbreviation c | Forms | 15S | Forms | 15R | Forms | 15S | Forms | 15R | |
| 1 | melocactin | 0.21 | ±0.027 | 0.08 | ±0.011 | 0.26 | ±0.041 | 0.12 | ±0.021 |
| 2 | hex-hex-Bd | 0.31 | ±0.039 | 0.11 | ±0.018 | 0.44 | ±0.071 | 0.15 | ±0.020 |
| 3 | hex-Bd | 3.7 | ±0.55 | 0.31 | ±0.048 | 17.5 | ±2.8 | 0.48 | ±0.074 |
| 4 | betanin | 0.37 | ±0.049 | 0.14 | ±0.020 | 0.44 | ±0.071 | 0.14 | ±0.020 |
| 5 | bougainvillein-v | 1.4 | ±0.20 | 1.1 | ±0.17 | 1.9 | ±0.27 | 1.5 | ±0.24 |
| 6 | hex-hex-Bd | 0.25 | ±0.033 | 0.061 | ±0.0090 | 0.44 | ±0.063 | 0.13 | ±0.017 |
| 7 | gomphrenin | 39.7 | ±2.8 | 13.7 | ±0.97 | 43.9 | ±2.2 | 13.2 | ±0.81 |
| 8a | 3’-mal-Gp | 0.18 | ±0.023 | 0.02 | ±0.0034 | ||||
| 8b | 4’-mal-Gp | 0.046 | ±0.0071 | 0.066 | ±0.010 | ||||
| 8c | 6’-mal-Gp | 3.9 | ±0.53 | 0.46 | ±0.071 | 0.79 | ±0.11 | 0.12 | ±0.018 |
| 9 | C9H8NO4-Gp | 2.0 | ±0.29 | 0.11 | ±0.018 | 2.2 | ±0.37 | 0.13 | ±0.021 |
| 10a | (3’’-OH-but)-hex-Bd | 0.24 | ±0.032 | 0.066 | ±0.010 | ||||
| 11 | C10H10NO5-Gp | 0.15 | ±0.023 | 0.006 | ±0.0009 | 0.19 | ±0.025 | 0.018 | ±0.0027 |
| 12 | hex-(coum-hex)-Bd | 0.71 | ±0.087 | 0.11 | ±0.018 | 0.17 | ±0.028 | 0.066 | ±0.010 |
| 10b | (3’’-OH-but)-hex-Bd | 0.63 | ±0.099 | 0.088 | ±0.013 | ||||
| 10c/d | (3’’-OH-but)-Gp | 4.4 | ±0.62 | 0.46 | ±0.074 | 0.61 | ±0.099 | 0.19 | ±0.023 |
| 13 | C8H6NO3-Gp | 0.49 | ±0.064 | 0.16 | ±0.16 | 0.35 | ±0.053 | 0.69 | ±0.10 |
| 14 | C7H8NO2-Gp | 2.3 | ±0.34 | 0.37 | ±0.059 | 0.70 | ±0.11 | 0.15 | ±0.019 |
| 15 | caff-Gp | 2.4 | ±0.31 | 0.42 | ±0.067 | 1.5 | ±0.22 | 0.26 | ±0.041 |
| 16 | Z-coum-Gp | 0.15 | ±0.023 | 0.052 | ±0.0085 | 0.15 | ±0.022 | 0.044 | ±0.0065 |
| 17 | Z-fer-Gp | 0.20 | ±0.027 | 0.015 | ±0.0026 | 0.64 | ±0.11 | 0.11 | ±0.014 |
| 18 | hex-(coum-hex)-Bd | 0.33 | ±0.038 | 0.24 | ±0.037 | 0.23 | ±0.035 | 0.17 | ±0.020 |
| 19 | sin-Gp | 0.46 | ±0.058 | 0.18 | ±0.026 | 0.57 | ±0.095 | 0.20 | ±0.027 |
| 20 | coum-Gp | 12.9 | ±1.8 | 1.8 | ±0.31 | 6.6 | ±1.1 | 0.82 | ±0.14 |
| 21 | fer-Gp | 2.1 | ±0.28 | 0.61 | ±0.098 | 0.21 | ±0.19 | 1.2 | ±0.035 |
| Total pigment: content [mg/100 g] b | 42.0 | ±0.25 | 86.6 | ±0.67 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutor-Świeży, K.; Antonik, M.; Dziedzic, E.; Bieniasz, M.; Mielczarek, P.; Popenda, Ł.; Pasternak, K.; Tyszka-Czochara, M.; Wybraniec, S. Structural Studies on Diverse Betacyanin Classes in Matured Pigment-Rich Fruits of Basella alba L. and Basella alba L. var. ‘Rubra’ (Malabar Spinach). Int. J. Mol. Sci. 2022, 23, 11243. https://doi.org/10.3390/ijms231911243
Sutor-Świeży K, Antonik M, Dziedzic E, Bieniasz M, Mielczarek P, Popenda Ł, Pasternak K, Tyszka-Czochara M, Wybraniec S. Structural Studies on Diverse Betacyanin Classes in Matured Pigment-Rich Fruits of Basella alba L. and Basella alba L. var. ‘Rubra’ (Malabar Spinach). International Journal of Molecular Sciences. 2022; 23(19):11243. https://doi.org/10.3390/ijms231911243
Chicago/Turabian StyleSutor-Świeży, Katarzyna, Michał Antonik, Ewa Dziedzic, Monika Bieniasz, Przemysław Mielczarek, Łukasz Popenda, Karol Pasternak, Małgorzata Tyszka-Czochara, and Sławomir Wybraniec. 2022. "Structural Studies on Diverse Betacyanin Classes in Matured Pigment-Rich Fruits of Basella alba L. and Basella alba L. var. ‘Rubra’ (Malabar Spinach)" International Journal of Molecular Sciences 23, no. 19: 11243. https://doi.org/10.3390/ijms231911243