Lipid-Based Nanoparticles as a Pivotal Delivery Approach in Triple Negative Breast Cancer (TNBC) Therapy
Abstract
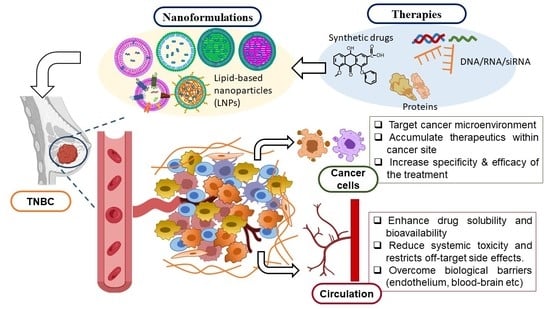
:1. Introduction
2. Lipid-Based Nanoparticles: A Versatile Drug Delivery System
2.1. Liposomes
2.2. Nanoemulsions (NEs)
2.3. Solid Lipid Nanoparticles (SLNs)
2.4. Nanostructured Lipid Carriers (NLCs)
2.5. Lipid Polymer Hybrid Nanoparticles (LPH-NPs)
2.6. Exosomes
3. Clinical Status
| S.No. | Brand Name | Formulation | Company of Manufacture | Use | Approval Year | Ref. |
|---|---|---|---|---|---|---|
| 1 | Doxil | Liposomal doxorubicin HCl (PEGylated) | Janssen | Kaposi’s sarcoma, ovarian cancer, multiple myeloma | 1995 | [107] |
| 2 | DaunoXome | Liposomal daunorubicin | Galen | Kaposi’s sarcoma | 1996 | |
| 3 | DepoCyt© | Liposomal cytarabine | Pacira Pharms Inc. | Lymphoma | 1996 | |
| 4 | Myocet | Liposomal doxorubicin (non-PEGylated) | Teva UK | Metastatic breast cancer | 2000 | [108] |
| 5 | MEPACT | Liposomal Mifamurtide | Takeda | Osteo-sarcoma | 2009 | [109] |
| 6 | Marqibo | Liposomal vincristine | Acrotech Biopharma | Acute lympho-blastic leukaemia | 2012 | [110] |
| 7 | Onivyde | Liposomal irinotecan | Ipsen | Metastatic pancreatic cancer | 2015 | [111] |
| 8 | Vyxeos | Liposome encapsulating Cytarabine: daunorubicin in fixed-dose | Jazz Pharmaceuticals | Acute myeloid leukemia | 2017 | [112] |
| S.No. | Cancer Type | LNPs | Route; Size | Status | Ref. |
|---|---|---|---|---|---|
| 1. | Glioblastoma | Curcumin-loaded NE | Oral; 67 ± 6 nm | In vitro and In vivo | [113] |
| Transferrin conjugated liposome encapsulating doxorubicin and erlotinib | 158.7–165.05 nm | In vitro | [114] | ||
| naI- IRI loaded liposome targeting topoisomerase I | Intravenous; 88–95 nm | Phase I clinical trial | [115] | ||
| Docetaxel-loaded SLN targeting LRP1 | Intravenous; 79–111.4 nm | In vitro and In vivo | [116] | ||
| Ferulic acid-loaded NLCs | <50 nm | In vitro | [117] | ||
| Lactoferrin and RGD peptide conjugated NLCs encapsulating temozolomide and vincristine | Intravenous; 96 nm | In vitro and In vivo | [118] | ||
| 2. | Esophageal | Rhenium loaded liposomes | Intravenous; <100 nm | In vitro and In vivo | [119] |
| LY294002 and 5-FU co-loaded Liposome (PEGylated) targeting thymidylate synthase | Intravenous; 110 nm | In vitro and In vivo | [120] | ||
| 3. | Lung | 9-bromo-noscapine-loaded NE | Inhalation; 13.4 ± 3.2 nm | In vitro and In vivo | [121] |
| Diferuloylmethane-loaded NE | Oral; ∼232.7 nm | In vitro and In vivo | [122] | ||
| PEG-lecithin and nRGD peptide conjugated NE-loaded lycobetaine and oleic acid | Intravenous; 158.42 ± 2.87 nm | In vitro and In vivo | [123] | ||
| Paclitaxel–Carboplatin–Gemcitabine-loaded liposome targeting tubulin | Percutaneous; 130 nm | Phase III clinical trial | [124] | ||
| miR-34a conjugated Paclitaxel-loaded SLNs | Intravenous; 218.2 nm | In vitro and In vivo | [125] | ||
| Transferrin-conjugated SLNs encapsulating Docetaxel and Baicalin | Intravenous; 135.5 nm | In vitro and In vivo | [126] | ||
| Gemcitabine and Paclitaxel co-loaded NLC with surface functionalized via glucose receptor-targeting ligand | 120.3 ± 1.3 nm | In vitro | [127] | ||
| 4. | Breast | Doxorubicin and bromotetrandrine (W198)-loaded NE | Intravenous; 99.5–152.6 nm | In vitro and In vivo | [128] |
| Doxorubicin and lapatinib-loaded liposome (PEGylated) | Intravenous; 100 nm | Phase Ib clinical trial | [129] | ||
| Hyaluronic acid-coated Paclitaxel-pDNA-loaded SLNs | Intravenous; 156.3 ± 5.5 nm | In vitro and In vivo | [130] | ||
| Fucose-conjugated Methotrexate-loaded SLNs | Intravenous; 174.51 ± 5.1 nm | In vitro and In vivo | [131] | ||
| Lapachone and Doxorubicin loaded NLCs | Intravenous; 100.2 ± 6.8 nm | In vitro and In vivo | [132] | ||
| 5. | Liver | Cantharidin-loaded liposomes (PEGylated) | Intravenous; 129.9 ± 2.5 nm | In vitro and In vivo | [133] |
| Glycyrrhetinic acid-functionalized curcumin-loaded liposomes | Intravenous; 194 ± 0.25 nm | In vitro and In vivo | [134] | ||
| miR-34a surface-functionalized liposomes | Intravenous; 120.21 ± 5 nm | Phase I clinical trial | [135] | ||
| Sorafenib-loaded SLNs | 248 ± 113 nm | In vitro | [136] | ||
| Paclitaxel-loaded NLCs | Oral; 153.8 ± 5.58 nm | In vitro and In vivo | [137] | ||
| 6. | Gastric | Indocyanine green-loaded liposome (PEGylated) | Intravenous; ~106 nm | In vitro and In vivo | [138] |
| CD44 antibody-conjugated SATB1 siRNA-loaded liposome | 159.3 nm | In vitro | [139] | ||
| Etoposide-loaded SLNs | 30–50 nm | In vitro | [140] | ||
| Sorafenib and miR-542-3p-loaded SLNs (PEGylated) | Intravenous; ~156 nm | In vitro and In vivo | [141] | ||
| Etoposide and curcumin co-loaded NLCs | Intravenous; 114 nm | In vitro and In vivo | [142] | ||
| 7. | Pancreatic | Gemcitabine-loaded NE | ~150 nm, | In vitro | [143] |
| Gemcitabine and synthetic curcumin (EF24) combined loaded liposomes (PEGylated) | Intravenous; < 150 nm | In vitro and In vivo | [144] | ||
| HSA-conjugated liposomes encapsulating Paclitaxel and Ellagic acid | Intravenous; 176.2 nm | In vitro and In vivo | [145] | ||
| naI-IRI, 5-FU, and Leucovorin co-loaded liposomes | Intravenous; <200 nm | Phase III clinical trial | [146] | ||
| 8. | Colorectal | Folic acid-conjugated 5-FU-loaded liposome | Intraperitoneal; 114 ± 4.58 nm | In vitro and In vivo | [147] |
| Omega 3-fatty acid (DHA) and resveratrol-loaded SLNs | 100 ± 1.8 nm | In vitro | [148] | ||
| Folic acid and dextran-conjugated SLNs encapsulating Doxorubicin | Oral; 99–144 nm | In vitro and In vivo | [149] | ||
| Hyaluronic acid-conjugated Irinotecan-loaded NLCs | 386 ± 2.2 nm | In vitro | [150] | ||
| 9. | Prostrate | Omega 3-fatty acid-conjugated Taxoid prodrug-loaded NE | Intravenous; 228 ± 7 nm | In vitro and In vivo | [151] |
| Catechin extract-loaded NE | 11.45 nm | In vitro | [152] | ||
| Oleuropein-loaded liposome (PEGylated) | Intravenous; 184.2 ± 9.16 nm | In vitro and In vivo | [153] | ||
| LRP1-targeted docetaxel-loaded liposome (PEGylated) | Intravenous; 163.2 ± 1.83 nm | In vitro and In vivo | [154] |
4. Toxicity of Lipid-Based Nanoparticles (LNPs)
5. Conclusions
6. Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data availability statement
Acknowledgments
Conflicts of Interest
References
- Gallo, C. Triple Negative Breast Cancer Epidemiology. 2018. Available online: https://oncologynurse-ce.com/triple-negative-breast-cancer-epidemiology (accessed on 1 April 2020).
- Aqil, F.; Munagala, R.; Agrawal, A.K.; Jeyabalan, J.; Tyagi, N.; Rai, S.N.; Gupta, R.C. Anthocyanidins Inhibit Growth and Chemosensitize Triple-Negative Breast Cancer via the NF-kappaB Signaling Pathway. Cancers 2021, 13, 6428. [Google Scholar]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for cancer therapy: Current progress and perspectives. J. Hematol. Oncol. 2021, 14, 85. [Google Scholar]
- El Moukhtari, S.H.; Rodriguez-Nogales, C.; Blanco-Prieto, M.J. Oral lipid nanomedicines: Current status and future perspectives in cancer treatment. Adv. Drug Deliv. Rev. 2021, 173, 238–251. [Google Scholar] [PubMed]
- Yan, L.; Shen, J.; Wang, J.; Yang, X.; Dong, S.; Lu, S. Nanoparticle-Based Drug Delivery System: A Patient-Friendly Chemotherapy for Oncology. Dose-Response 2020, 18, 1559325820936161. [Google Scholar]
- Miller, A.D. Lipid-based nanoparticles in cancer diagnosis and therapy. J. Drug Deliv. 2013, 2013, 165981. [Google Scholar]
- Aqil, F.; Munagala, R.; Agrawal, A.K.; Gupta, R. Anticancer Phytocompounds. In New Look to Phytomedicine; Elsevier Inc.: Cambridge, MA, USA, 2019; pp. 237–272. [Google Scholar]
- Talluri, S.V.; Kuppusamy, G.; Karri, V.V.S.R.; Tummala, S.; Madhunapantula, S. Lipid-based nanocarriers for breast cancer treatment-comprehensive review. Drug Deliv. 2016, 23, 1291–1305. [Google Scholar]
- Chaudhuri, A.; Kumar, D.N.; Dehari, D.; Singh, S.; Kumar, P.; Bolla, P.K.; Kumar, D.; Agrawal, A.K. Emergence of Nanotechnology as a Powerful Cavalry against Triple-Negative Breast Cancer (TNBC). Pharmaceuticals 2022, 15, 542. [Google Scholar]
- Negi, S.; Chaudhuri, A.; Kumar, D.N.; Dehari, D.; Singh, S.; Agrawal, A.K. Nanotherapeutics in autophagy: A paradigm shift in cancer treatment. Drug Deliv. Transl. Res. 2022, 1–24. [Google Scholar] [CrossRef]
- Jain, S.; Spandana, G.; Agrawal, A.K.; Kushwah, V.; Thanki, K. Enhanced Antitumor Efficacy and Reduced Toxicity of Docetaxel Loaded Estradiol Functionalized Stealth Polymeric Nanoparticles. Mol. Pharm. 2015, 12, 3871–3884. [Google Scholar]
- Agrawal, A.K.; Kumar, K.; Swarnakar, N.K.; Kushwah, V.; Jain, S. “Liquid Crystalline Nanoparticles”: Rationally Designed Vehicle To Improve Stability and Therapeutic Efficacy of Insulin Following Oral Administration. Mol. Pharm. 2017, 14, 1874–1882. [Google Scholar]
- Karunanidhi, P.; Verma, N.; Kumar, D.N.; Agrawal, A.K.; Singh, S. Triphenylphosphonium functionalized Ficus religiosa L. extract loaded nanoparticles improve the mitochondrial function in oxidative stress induced diabetes. AAPS PharmSciTech 2021, 22, 158. [Google Scholar]
- Singh, S.; Kushwah, V.; Agrawal, A.K.; Jain, S. Insulin- and quercetin-loaded liquid crystalline nanoparticles: Implications on oral bioavailability, antidiabetic and antioxidant efficacy. Nanomedicine 2018, 13, 521–537. [Google Scholar] [PubMed]
- Urimi, D.; Agrawal, A.K.; Kushwah, V.; Jain, S. Polyglutamic Acid Functionalization of Chitosan Nanoparticles Enhances the Therapeutic Efficacy of Insulin Following Oral Administration. AAPS PharmSciTech 2019, 20, 131. [Google Scholar]
- Anjum, M.M.; Patel, K.K.; Dehari, D.; Pandey, N.; Tilak, R.; Agrawal, A.K.; Singh, S. Anacardic acid encapsulated solid lipid nanoparticles for Staphylococcus aureus biofilm therapy: Chitosan and DNase coating improves antimicrobial activity. Drug Deliv. Transl. Res. 2021, 11, 305–317. [Google Scholar] [PubMed]
- Patel, K.K.; Agrawal, A.K.; Anjum, M.; Tripathi, M.; Pandey, N.; Bhattacharya, S.; Tilak, R.; Singh, S. DNase-I functionalization of ciprofloxacin-loaded chitosan nanoparticles overcomes the biofilm-mediated resistance of Pseudomonas aeruginosa. Appl. Nanosci. 2019, 10, 563–575. [Google Scholar]
- Patel, K.K.; Surekha, D.B.; Tripathi, M.; Anjum, M.M.; Muthu, M.S.; Tilak, R.; Agrawal, A.K.; Singh, S. Antibiofilm Potential of Silver Sulfadiazine-Loaded Nanoparticle Formulations: A Study on the Effect of DNase-I on Microbial Biofilm and Wound Healing Activity. Mol. Pharm. 2019, 16, 3916–3925. [Google Scholar]
- Harde, H.; Agrawal, A.K.; Jain, S. Development of stabilized glucomannosylated chitosan nanoparticles using tandem crosslinking method for oral vaccine delivery. Nanomedicine 2014, 9, 2511–2599. [Google Scholar]
- Harde, H.; Agrawal, A.K.; Jain, S. Tetanus toxoids loaded glucomannosylated chitosan based nanohoming vaccine adjuvant with improved oral stability and immunostimulatory response. Pharm. Res. 2015, 32, 122–134. [Google Scholar]
- Rai, V.K.; Mishra, N.; Agrawal, A.; Jain, S.; Yadav, N.P. Novel drug delivery system: An immense hope for diabetics. Drug Deliv. 2016, 23, 2371–2390. [Google Scholar] [PubMed]
- Dehari, D.; Chaudhuri, A.; Singh, S.; Agrawal, A.K. RNA-Based Vaccines for Infectious Disease; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Jain, S.; Kumar, S.; Agrawal, A.K.; Thanki, K.; Banerjee, U.C. Hyaluronic acid–PEI–cyclodextrin polyplexes: Implications for in vitro and in vivo transfection efficiency and toxicity. RSC Adv. 2015, 5, 41144–41154. [Google Scholar]
- Jain, S.; Raza, K.; Agrawal, A.K.; Vaidya, A. Nanotechnology Applications for Cancer Chemotherapy; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Cerpnjak, K.; Zvonar, A.; Gašperlin, M.; Vrečer, F. Lipid-based systems as a promising approach for enhancing the bioavailability of poorly water-soluble drugs. Acta Pharm. 2013, 63, 427–445. [Google Scholar] [PubMed]
- Kushwah, V.; Katiyar, S.S.; Agrawal, A.K.; Gupta, R.C.; Jain, S. Co-delivery of docetaxel and gemcitabine using PEGylated self-assembled stealth nanoparticles for improved breast cancer therapy. Nanomedicine 2018, 14, 1629–1641. [Google Scholar] [PubMed]
- Kushwah, V.; Katiyar, S.S.; Agrawal, A.K.; Saraf, I.; Singh, I.P.; Lamprou, D.A.; Gupta, R.C.; Jain, S. Implication of linker length on cell cytotoxicity, pharmacokinetic and toxicity profile of gemcitabine-docetaxel combinatorial dual drug conjugate. Int. J. Pharm. 2018, 548, 357–374. [Google Scholar]
- Lim, S.B.; Banerjee, A.; Onyuksel, H. Improvement of drug safety by the use of lipid-based nanocarriers. J. Control. Release 2012, 163, 34–45. [Google Scholar] [PubMed]
- Palei, N.N.; Mohanta, B.C.; Sabapathi, M.L.; Das, M.K. Lipid-based nanoparticles for cancer diagnosis and therapy. In Organic Materials as Smart Nanocarriers for Drug Delivery; Elsevier Inc.: Cambridge, MA, USA, 2018; pp. 415–470. [Google Scholar]
- Porter, C.J.; Trevaskis, N.L.; Charman, W.N. Lipids and lipid-based formulations: Optimizing the oral delivery of lipophilic drugs. Nat. Rev. Drug Discov. 2007, 6, 231–248. [Google Scholar] [PubMed]
- Mohsin, K.; Shahba, A.A.; Alanazi, F.K. Lipid Based Self Emulsifying Formulations for Poorly Water Soluble Drugs-An Excellent Opportunity. Indian J. Pharm. Educ. Res. 2012, 46, 88–96. [Google Scholar]
- Ali Khan, A.; Khan, A.A.; Mudassir, J.; Mohtar, N. Advanced drug delivery to the lymphatic system: Lipid-based nanoformulations. Int. J. Nanomed. 2013, 8, 2733–2744. [Google Scholar]
- Qi, J.; Zhuang, J.; Lu, Y.; Dong, X.; Zhao, W.; Wu, W. In vivo fate of lipid-based nanoparticles. Drug Discov. Today 2017, 22, 166–172. [Google Scholar]
- Kim, M.W.; Kwon, S.-H.; Choi, J.H.; Lee, A. A Promising Biocompatible Platform: Lipid-Based and Bio-Inspired Smart Drug Delivery Systems for Cancer Therapy. Int. J. Mol. Sci. 2018, 19, 3859. [Google Scholar]
- Paliwal, R.; Babu, R.J.; Palakurthi, S. Nanomedicine scale-up technologies: Feasibilities and challenges. AAPS PharmSciTech 2014, 15, 1527–1534. [Google Scholar]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.; Lillard, J.W., Jr. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Mendes, B.B.; Conniot, J.; Avital, A.; Yao, D.; Jiang, X.; Zhou, X.; Sharf-Pauker, N.; Xiao, Y.; Adir, O.; Liang, H.; et al. Nanodelivery of nucleic acids. Nat. Rev. Methods Prim. 2022, 2, 25. [Google Scholar] [CrossRef]
- Kumar, D.N.; Chaudhuri, A.; Aqil, F.; Dehari, D.; Munagala, R.; Singh, S.; Gupta, R.C.; Agrawal, A.K. Exosomes as Emerging Drug Delivery and Diagnostic Modality for Breast Cancer: Recent Advances in Isolation and Application. Cancers 2022, 14, 1435. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.A.; Shmeeda, H.; Zalipsky, S. Pros and cons of the liposome platform in cancer drug targeting. J. Liposome Res. 2006, 16, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef]
- Kaur, T.; Slavcev, R. Solid Lipid Nanoparticles: Tuneable Anti-Cancer Gene/Drug Delivery Systems. In Novel Gene Therapy Approaches; IntechOpen: London, UK, 2013. [Google Scholar]
- Iqbal, M.A.; Md, S.; Sahni, J.K.; Baboota, S.; Dang, S.; Ali, J. Nanostructured lipid carriers system: Recent advances in drug delivery. J. Drug Target. 2012, 20, 813–830. [Google Scholar] [CrossRef] [PubMed]
- Persano, F.; Gigli, G.; Leporatti, S. Lipid-polymer hybrid nanoparticles in cancer therapy: Current overview and future directions. Nano Express 2021, 2, 012006. [Google Scholar] [CrossRef]
- Antimisiaris, S.G.; Mourtas, S.; Marazioti, A. Exosomes and Exosome-Inspired Vesicles for Targeted Drug Delivery. Pharmaceutics 2018, 10, 218. [Google Scholar] [CrossRef]
- Chen, Y.S.; Chen, Y.-S.; Lin, E.-Y.; Chiou, T.-W. Exosomes in clinical trial and their production in compliance with good manufacturing practice. Tzu Chi Med. J. 2020, 32, 113–120. [Google Scholar]
- Arias, J.L.; Clares, B.; Morales, M.E.; Gallardo, V.; Ruiz, M.A. Lipid-Based Drug Delivery Systems for Cancer Treatment. Curr. Drug Targets 2011, 12, 1151–1165. [Google Scholar] [CrossRef] [PubMed]
- Kushwah, V.; Jain, D.K.; Agrawal, A.K.; Jain, S. Improved antitumor efficacy and reduced toxicity of docetaxel using anacardic acid functionalized stealth liposomes. Coll. Surf. B Biointerfaces 2018, 172, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Harde, H.; Indulkar, A.; Agrawal, A. Improved stability and immunological potential of tetanus toxoid containing surface engineered bilosomes following oral administration. Nanomedicine 2014, 10, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Patil, S.R.; Swarnakar, N.K.; Agrawal, A.K. Oral delivery of doxorubicin using novel polyelectrolyte-stabilized liposomes (layersomes). Mol. Pharm. 2012, 9, 2626–2635. [Google Scholar] [CrossRef] [PubMed]
- Olusanya, T.O.B.; Haj Ahmad, R.R.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal Drug Delivery Systems and Anticancer Drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef]
- Guo, P.; Yang, J.; Liu, D.; Huang, L.; Fell, G.; Huang, J.; Moses, M.A.; Auguste, D.T. Dual complementary liposomes inhibit triple-negative breast tumor progression and metastasis. Sci. Adv. 2019, 5, eaav5010. [Google Scholar] [CrossRef]
- Yan, Y.; Li, X.-Q.; Duan, J.-L.; Bao, C.-J.; Cui, Y.-N.; Su, Z.-B.; Xu, J.-R.; Luo, Q.; Chen, M.; Xie, Y.; et al. Nanosized functional miRNA liposomes and application in the treatment of TNBC by silencing Slug gene. Int. J. Nanomed. 2019, 14, 3645–3667. [Google Scholar] [CrossRef]
- Chen, M.; Miao, Y.; Qian, K.; Zhou, X.; Guo, L.; Qiu, Y.; Wang, R.; Gan, Y.; Zhang, X. Detachable Liposomes Combined Immunochemotherapy for Enhanced Triple-Negative Breast Cancer Treatment through Reprogramming of Tumor-Associated Macrophages. Nano Lett. 2021, 21, 6031–6041. [Google Scholar] [CrossRef]
- Alawak, M.; Abu Dayyih, A.; Mahmoud, G.; Tariq, I.; Duse, L.; Goergen, N.; Engelhardt, K.; Pinnapireddy, S.R.; Jedelská, J.; Awak, M.; et al. ADAM 8 as a novel target for doxorubicin delivery to TNBC cells using magnetic thermosensitive liposomes. Eur. J. Pharm. Biopharm. 2021, 158, 390–400. [Google Scholar] [CrossRef]
- El-Senduny, F.F.; Altouhamy, M.; Zayed, G.; Harsha, C.; Jalaja, R.; Somappa, S.B.; Nair, M.S.; Kunnumakkara, A.B.; Alsharif, F.M.; Badria, F.A. Azadiradione-loaded liposomes with improved bioavailability and anticancer efficacy against triple negative breast cancer. J. Drug Deliv. Sci. Technol. 2021, 65, 102665. [Google Scholar] [CrossRef]
- Souto, E.B.; Nayak, A.B.; Murthy, R.S.R. Lipid nanoemulsions for anti-cancer drug therapy. Pharmazie 2011, 66, 473–478. [Google Scholar] [PubMed]
- Jain, S.; Garg, T.; Kushwah, V.; Thanki, K.; Agrawal, A.K.; Dora, C.P. alpha-Tocopherol as functional excipient for resveratrol and coenzyme Q10-loaded SNEDDS for improved bioavailability and prophylaxis of breast cancer. J. Drug Target. 2017, 25, 554–565. [Google Scholar] [CrossRef]
- Gawin-Mikolajewicz, A.; Nartowski, K.P.; Dyba, A.J.; Gołkowska, A.M.; Malec, K.; Karolewicz, B. Ophthalmic Nanoemulsions: From Composition to Technological Processes and Quality Control. Mol. Pharm. 2021, 18, 3719–3740. [Google Scholar] [CrossRef] [PubMed]
- Ganta, S.; Talekar, M.; Singh, A.; Coleman, T.P.; Amiji, M.M. Nanoemulsions in translational research-opportunities and challenges in targeted cancer therapy. AAPS PharmSciTech 2014, 15, 694–708. [Google Scholar] [CrossRef]
- Sanchez-Lopez, E.; Guerra, M.; Dias-Ferreira, J.; Lopez-Machado, A.; Ettcheto, M.; Cano, A.; Espina, M.; Camins, A.; Garcia, M.L.; Souto, E.B. Current Applications of Nanoemulsions in Cancer Therapeutics. Nanomaterials 2019, 9, 821. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Pena, C.D.; Auguste, D.T. Targeted Lipid Nanoemulsions Encapsulating Epigenetic Drugs Exhibit Selective Cytotoxicity on CDH1−/FOXM1+ Triple Negative Breast Cancer Cells. Mol. Pharm. 2019, 16, 1813–1826. [Google Scholar] [CrossRef]
- Xu, H.; Hu, M.; Liu, M.; An, S.; Guan, K.; Wang, M.; Li, L.; Zhang, J.; Li, J.; Huang, L. Nano-puerarin regulates tumor microenvironment and facilitates chemo- and immunotherapy in murine triple negative breast cancer model. Biomaterials 2020, 235, 119769. [Google Scholar] [CrossRef]
- Han, B.; Wang, T.; Xue, Z.; Wen, T.; Lu, L.; Meng, J.; Liu, J.; Wu, S.; Yu, J.; Xu, H. Elemene Nanoemulsion Inhibits Metastasis of Breast Cancer by ROS Scavenging. Int. J. Nanomed. 2021, 16, 6035–6048. [Google Scholar] [CrossRef]
- Saraiva, S.M.; Gutiérrez-Lovera, C.; Martínez-Val, J.; Lores, S.; Bouzo, B.L.; Díez-Villares, S.; Alijas, S.; Pensado-López, A.; Vázquez-Ríos, A.J.; Sánchez, L.; et al. Edelfosine nanoemulsions inhibit tumor growth of triple negative breast cancer in zebrafish xenograft model. Sci. Rep. 2021, 11, 9873. [Google Scholar] [CrossRef]
- Hallan, S.S.; Sguizzato, M.; Esposito, E.; Cortesi, R. Challenges in the Physical Characterization of Lipid Nanoparticles. Pharmaceutics 2021, 13, 549. [Google Scholar] [CrossRef]
- Patel, K.K.; Gade, S.; Anjum, M.; Singh, S.K.; Maiti, P.; Agrawal, A.K. Effect of penetration enhancers and amorphization on transdermal permeation flux of raloxifene-encapsulated solid lipid nanoparticles: An ex vivo study on human skin. Appl. Nanosci. 2019, 9, 1383–1394. [Google Scholar] [CrossRef]
- Parvez, S.; Yadagiri, G.; Gedda, M.R.; Singh, A.; Singh, O.P.; Verma, A.; Sundar, S.; Mudavath, S.L. Modified solid lipid nanoparticles encapsulated with Amphotericin B and Paromomycin: An effective oral combination against experimental murine visceral leishmaniasis. Sci. Rep. 2020, 10, 12243. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Yadagiri, G.; Parvez, S.; Singh, O.P.; Verma, A.; Sundar, S.; Mudavath, S.L. Formulation, characterization and in vitro anti-leishmanial evaluation of amphotericin B loaded solid lipid nanoparticles coated with vitamin B12-stearic acid conjugate. Mater. Sci. Eng. C 2020, 117, 111279. [Google Scholar] [CrossRef]
- Harde, H.; Agrawal, A.K.; Katariya, M.; Kale, D.; Jain, S. Development of a topical adapalene-solid lipid nanoparticle loaded gel with enhanced efficacy and improved skin tolerability. RSC Adv. 2015, 5, 43917–43929. [Google Scholar] [CrossRef]
- Edis, Z.; Wang, J.; Waqas, M.K.; Ijaz, M.; Ijaz, M. Nanocarriers-Mediated Drug Delivery Systems for Anticancer Agents: An Overview and Perspectives. Int. J. Nanomed. 2021, 16, 1313–1330. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Dhar, A.; Patel, C.; Khimani, M.; Neogi, S.; Sharma, P.; Kumar, N.S.; Vekariya, R.L. A brief review on solid lipid nanoparticles: Part and parcel of contemporary drug delivery systems. RSC Adv. 2020, 10, 26777–26791. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.L.; Bendayan, R.; Rauth, A.M.; Li, Y.; Wu, X.Y. Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Adv. Drug Deliv. Rev. 2007, 59, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Puri, A.; Loomis, K.; Smith, B.; Lee, J.H.; Yavlovich, A.; Heldman, E.; Blumenthal, R. Lipid-Based Nanoparticles as Pharmaceutical Drug Carriers: From Concepts to Clinic. Crit. Rev. Ther. Drug Carrier Syst. 2009, 26, 523–580. [Google Scholar] [CrossRef]
- Guney Eskiler, G.; Cecener, G.; Egeli, U.; Tunca, B. Synthetically Lethal BMN 673 (Talazoparib) Loaded Solid Lipid Nanoparticles for BRCA1 Mutant Triple Negative Breast Cancer. Pharm. Res. 2018, 35, 218. [Google Scholar] [CrossRef]
- Siddhartha, V.T.; Pindiprolu, S.K.S.S.; Chintamaneni, P.K.; Tummala, S.; Kumar, S.N. RAGE receptor targeted bioconjuguate lipid nanoparticles of diallyl disulfide for improved apoptotic activity in triple negative breast cancer: In vitro studies. Artif. Cells Nanomed. Biotechnol. 2018, 46, 387–397. [Google Scholar] [CrossRef]
- Kothari, I.R.; Mazumdar, S.; Sharma, S.; Italiya, K.; Mittal, A.; Chitkara, D. Docetaxel and alpha-lipoic acid co-loaded nanoparticles for cancer therapy. Ther. Deliv. 2019, 10, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Pindiprolu, S.; Chintamaneni, P.K.; Krishnamurthy, P.T.; Ratna Sree Ganapathineedi, K. Formulation-optimization of solid lipid nanocarrier system of STAT3 inhibitor to improve its activity in triple negative breast cancer cells. Drug Dev. Ind. Pharm. 2019, 45, 304–313. [Google Scholar] [CrossRef]
- Pindiprolu, S.K.S.S.; Krishnamurthy, P.T.; Ghanta, V.R.; Chintamaneni, P.K. Phenyl boronic acid-modified lipid nanocarriers of niclosamide for targeting triple-negative breast cancer. Nanomedicine 2020, 15, 1551–1565. [Google Scholar] [CrossRef] [PubMed]
- Gade, S.; Patel, K.K.; Gupta, C.; Anjum, M.; Deepika, D.; Agrawal, A.K.; Singh, S. An Ex Vivo Evaluation of Moxifloxacin Nanostructured Lipid Carrier Enriched In Situ Gel for Transcorneal Permeation on Goat Cornea. J. Pharm. Sci. 2019, 108, 2905–2916. [Google Scholar] [CrossRef] [PubMed]
- Pedro, I.D.R.; Almeida, O.P.; Martins, H.R.; Lemos, J.D.A.; de Barros, A.L.; Leite, E.A.; Carneiro, G. Optimization and in vitro/in vivo performance of paclitaxel-loaded nanostructured lipid carriers for breast cancer treatment. J. Drug Deliv. Sci. Technol. 2019, 54, 101370. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, J.; Hu, H.; Yan, Y.; Hu, X.; Zhou, K.; Xiao, S.; Zhang, Y.T.; Feng, N. Construction and in vitro and in vivo evaluation of folic acid-modified nanostructured lipid carriers loaded with paclitaxel and chlorin e6. Int. J. Pharm. 2019, 569, 118595. [Google Scholar] [CrossRef]
- Lages, E.B.; Fernandes, R.S.; Silva, J.O.; de Souza, Â.M.; Cassali, G.D.; de Barros, A.L.B.; Miranda Ferreira, L.A. Co-delivery of doxorubicin, docosahexaenoic acid, and alpha-tocopherol succinate by nanostructured lipid carriers has a synergistic effect to enhance antitumor activity and reduce toxicity. Biomed. Pharmacother. 2020, 132, 110876. [Google Scholar] [CrossRef] [PubMed]
- Gadag, S.; Narayan, R.; Nayak, A.S.; Ardila, D.C.; Sant, S.; Nayak, Y.; Garg, S.; Nayak, U.Y. Development and preclinical evaluation of microneedle-assisted resveratrol loaded nanostructured lipid carriers for localized delivery to breast cancer therapy. Int. J. Pharm. 2021, 606, 120877. [Google Scholar] [CrossRef]
- Gilani, S.J.; Bin-Jumah, M.; Rizwanullah; Imam, S.; Imtiyaz, K.; Alshehri, S.; Rizvi, M. Chitosan Coated Luteolin Nanostructured Lipid Carriers: Optimization, In Vitro-Ex Vivo Assessments and Cytotoxicity Study in Breast Cancer Cells. Coatings 2021, 11, 158. [Google Scholar] [CrossRef]
- Jain, S.; Kumar, S.; Agrawal, A.K.; Thanki, K.; Banerjee, U.C. Enhanced transfection efficiency and reduced cytotoxicity of novel lipid-polymer hybrid nanoplexes. Mol. Pharm. 2013, 10, 2416–2425. [Google Scholar] [CrossRef]
- Zhang, T.; Prasad, P.; Cai, P.; He, C.; Shan, D.; Rauth, A.M.; Wu, X.Y. Dual-targeted hybrid nanoparticles of synergistic drugs for treating lung metastases of triple negative breast cancer in mice. Acta Pharmacol. Sin. 2017, 38, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Kennell, C.; Lee, J.Y.; Leung, Y.K.; Tarapore, P. Calcium phosphate-polymer hybrid nanoparticles for enhanced triple negative breast cancer treatment via co-delivery of paclitaxel and miR-221/222 inhibitors. Nanomedicine 2017, 13, 403–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, N.K.; Tyagi, R.K.; Sharma, G.; Jain, A.; Singh, B.; Jain, S.; Katare, O.P. Functionalized Lipid-Polymer Hybrid Nanoparticles Mediated Codelivery of Methotrexate and Aceclofenac: A Synergistic Effect in Breast Cancer with Improved Pharmacokinetics Attributes. Mol. Pharm. 2017, 14, 1883–1897. [Google Scholar] [CrossRef] [PubMed]
- Bakar-Ates, F.; Ozkan, E.; Sengel-Turk, C.T. Encapsulation of cucurbitacin B into lipid polymer hybrid nanocarriers induced apoptosis of MDAMB231 cells through PARP cleavage. Int. J. Pharm. 2020, 586, 119565. [Google Scholar] [CrossRef]
- Agrawal, A.K.; Aqil, F.; Jeyabalan, J.; Spencer, W.A.; Beck, J.; Gachuki, B.W.; Alhakeem, S.S.; Oben, K.; Munagala, R.; Bondada, S.; et al. Milk-derived exosomes for oral delivery of paclitaxel. Nanomedicine 2017, 13, 1627–1636. [Google Scholar] [CrossRef]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Agrawal, A.K.; Kyakulaga, A.-H.; Wilcher, S.A.; Gupta, R.C. Milk exosomes-Natural nanoparticles for siRNA delivery. Cancer Lett. 2019, 449, 186–195. [Google Scholar] [CrossRef]
- Kandimalla, R.; Aqil, F.; Alhakeem, S.; Jeyabalan, J.; Tyagi, N.; Agrawal, A.; Yan, J.; Spencer, W.; Bondada, S.; Gupta, R. Targeted Oral Delivery of Paclitaxel Using Colostrum-Derived Exosomes. Cancers 2021, 13, 3700. [Google Scholar] [CrossRef]
- Aqil, F.; Jeyabalan, J.; Agrawal, A.K.; Kyakulaga, A.-H.; Munagala, R.; Parker, L.; Gupta, R.C. Exosomal delivery of berry anthocyanidins for the management of ovarian cancer. Food Funct. 2017, 8, 4100–4107. [Google Scholar] [CrossRef]
- Aqil, F.; Kausar, H.; Agrawal, A.K.; Jeyabalan, J.; Kyakulaga, A.-H.; Munagala, R.; Gupta, R. Exosomal formulation enhances therapeutic response of celastrol against lung cancer. Exp. Mol. Pathol. 2016, 101, 12–21. [Google Scholar] [CrossRef]
- Anjum, M.M.; Kumar, D.N.; Chaudhuri, A.; Singh, S.; Agrawal, A.K. Extracellular Vesicles for Nucleic Acid Delivery-Progress and Prospects for Safe RNA-Based Gene Therapy, 1st ed.; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Kumar, D.N.; Chaudhuri, A.; Dehari, D.; Shekher, A.; Gupta, S.C.; Majumdar, S.; Krishnamurthy, S.; Singh, S.; Kumar, D.; Agrawal, A.K. Combination Therapy Comprising Paclitaxel and 5-Fluorouracil by Using Folic Acid Functionalized Bovine Milk Exosomes Improves the Therapeutic Efficacy against Breast Cancer. Life 2022, 12, 1143. [Google Scholar] [CrossRef]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Agrawal, A.K.; Mudd, A.M.; Kyakulaga, A.H.; Singh, I.P.; Vadhanam, M.V.; Gupta, R.C. Exosomal formulation of anthocyanidins against multiple cancer types. Cancer Lett. 2017, 393, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Agrawal, A.; Gupta, R. Exosomes for the Enhanced Tissue Bioavailability and Efficacy of Curcumin. AAPS J. 2017, 19, 1691–1702. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, C.; Huang, X.; Li, J.; Fu, Z.; Li, W.; Yin, Y. The Advancing Roles of Exosomes in Breast Cancer. Front. Cell Dev. Biol. 2021, 9, 731062. [Google Scholar] [CrossRef] [PubMed]
- Naseri, Z.; Oskuee, R.K.; Jaafari, M.R.; Forouzandeh, M. Exosome-mediated delivery of functionally active miRNA-142–3p inhibitor reduces tumorigenicity of breast cancer in vitro and in vivo. Int. J. Nanomed. 2018, 13, 7727–7747. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Tian, J.; Wang, Z.; Gao, Y.; Wu, X.; Ding, X.; Qiang, L.; Li, G.; Han, Z.; Yuan, Y.; et al. Functional exosome-mediated co-delivery of doxorubicin and hydrophobically modified microRNA 159 for triple-negative breast cancer therapy. J. Nanobiotechnol. 2019, 17, 93. [Google Scholar] [CrossRef]
- Yu, M.; Gai, C.; Li, Z.; Ding, D.; Zheng, J.; Zhang, W.; Lv, S.; Li, W. Targeted exosome-encapsulated erastin induced ferroptosis in triple negative breast cancer cells. Cancer Sci. 2019, 110, 3173–3182. [Google Scholar] [CrossRef]
- Li, S.; Wu, Y.; Ding, F.; Yang, J.; Li, J.; Gao, X.; Zhang, C.; Feng, J. Engineering macrophage-derived exosomes for targeted chemotherapy of triple-negative breast cancer. Nanoscale 2020, 12, 10854–10862. [Google Scholar] [CrossRef]
- Thi, T.T.H.; Suys, E.; Lee, J.; Nguyen, D.; Park, K.; Truong, N. Lipid-Based Nanoparticles in the Clinic and Clinical Trials: From Cancer Nanomedicine to COVID-19 Vaccines. Vaccines 2021, 9, 359. [Google Scholar] [CrossRef]
- Church, J.W. Celsion Provides Update on ThermoDox® in the Phase III OPTIMA Study of Primary Liver Cancer; Celsion Corporation: Lawrenceville, NJ, USA, 2022. [Google Scholar]
- Choi, Y.H.; Han, H.K. Nanomedicines: Current status and future perspectives in aspect of drug delivery and pharmacokinetics. J. Pharm. Investig. 2018, 48, 43–60. [Google Scholar] [CrossRef]
- Shah, S.; Dhawan, V.; Holm, R.; Nagarsenker, M.S.; Perrie, Y. Liposomes: Advancements and innovation in the manufacturing process. Adv. Drug Deliv. Rev. 2020, 154–155, 102–122. [Google Scholar] [CrossRef]
- Venkatakrishnan, K.; Liu, Y.; Noe, D.; Mertz, J.; Bargfrede, M.; Marbury, T.; Farbakhsh, K.; Oliva, C.; Milton, A. Pharmacokinetics and pharmacodynamics of liposomal mifamurtide in adult volunteers with mild or moderate renal impairment. Br. J. Clin. Pharmacol. 2014, 77, 986–997. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.A.; Deitcher, S.R. Marqibo(R) (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother. Pharmacol. 2013, 71, 555–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H. Onivyde for the therapy of multiple solid tumors. Onco Targets Ther. 2016, 9, 3001–3007. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef]
- Kumar, A.; Ahuja, A.; Ali, J.; Baboota, S. Curcumin-loaded lipid nanocarrier for improving bioavailability, stability and cytotoxicity against malignant glioma cells. Drug Deliv. 2016, 23, 214–229. [Google Scholar] [CrossRef]
- Lakkadwala, S.; Singh, J. Co-delivery of doxorubicin and erlotinib through liposomal nanoparticles for glioblastoma tumor regression using an in vitro brain tumor model. Coll. Surf. B Biointerfaces 2019, 173, 27–35. [Google Scholar] [CrossRef]
- Clarke, J.L.; Molinaro, A.M.; Cabrera, J.R.; DeSilva, A.A.; Rabbitt, J.E.; Prey, J.; Drummond, D.C.; Kim, J.; Noble, C.; Fitzgerald, J.B.; et al. A phase 1 trial of intravenous liposomal irinotecan in patients with recurrent high-grade glioma. Cancer Chemother. Pharmacol. 2017, 79, 603–610. [Google Scholar] [CrossRef]
- Kadari, A.; Pooja, D.; Gora, R.H.; Gudem, S.; Kolapalli, V.R.M.; Kulhari, H.; Sistla, R. Design of multifunctional peptide collaborated and docetaxel loaded lipid nanoparticles for antiglioma therapy. Eur. J. Pharm. Biopharm. 2018, 132, 168–179. [Google Scholar] [CrossRef]
- Carbone, C.; Campisi, A.; Musumeci, T.; Raciti, G.; Bonfanti, R.; Puglisi, G. FA-loaded lipid drug delivery systems: Preparation, characterization and biological studies. Eur. J. Pharm. Sci. 2014, 52, 12–20. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, X.; Zhu, J.; Gao, Z.; Lai, X.; Zhu, X.; Mao, G. Lactoferrin- and RGD-comodified, temozolomide and vincristine-coloaded nanostructured lipid carriers for gliomatosis cerebri combination therapy. Int. J. Nanomed. 2018, 13, 3039–3051. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Chang, C.H.; Chi, C.-W.; Yu, H.-L.; Chang, T.-J.; Tsai, T.-H.; Lee, T.-W.; Liu, S.-Y. External beam radiotherapy synergizes 188Re-liposome against human esophageal cancer xenograft and modulates 188Re-liposome pharmacokinetics. Int. J. Nanomed. 2015, 10, 3641–3649. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Gao, Y.; Wang, D.; Xu, Z.; Sun, W.; Ren, P. Autophagy Inhibitor (LY294002) and 5-fluorouracil (5-FU) Combination-Based Nanoliposome for Enhanced Efficacy Against Esophageal Squamous Cell Carcinoma. Nanoscale Res. Lett. 2018, 13, 325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jyoti, K.; Kaur, K.; Pandey, R.S.; Jain, U.K.; Chandra, R.; Madan, J. Inhalable nanostructured lipid particles of 9-bromo-noscapine, a tubulin-binding cytotoxic agent: In vitro and in vivo studies. J. Colloid Interface Sci. 2015, 445, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wan, K.; Hu, X.; Zhang, Y.; Yan, Z.; Feng, J.; Zhang, J. Functional nanoemulsion-hybrid lipid nanocarriers enhance the bioavailability and anti-cancer activity of lipophilic diferuloylmethane. Nanotechnology 2016, 27, 085102. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Gong, T.; Zhao, T.; Fu, Y.; Zhang, Z.; Gong, T. A comparison study between lycobetaine-loaded nanoemulsion and liposome using nRGD as therapeutic adjuvant for lung cancer therapy. Eur. J. Pharm. Sci. 2018, 111, 293–302. [Google Scholar] [CrossRef]
- Lu, B.; Sun, L.; Yan, X.; Ai, Z.; Xu, J. Intratumoral chemotherapy with paclitaxel liposome combined with systemic chemotherapy: A new method of neoadjuvant chemotherapy for stage III unresectable non-small cell lung cancer. Med. Oncol. 2015, 32, 345. [Google Scholar] [CrossRef]
- Shi, S.; Han, L.; Deng, L.; Zhang, Y.; Shen, H.; Gong, T.; Zhang, Z.; Sun, X. Dual drugs (microRNA-34a and paclitaxel)-loaded functional solid lipid nanoparticles for synergistic cancer cell suppression. J. Control. Release 2014, 194, 228–237. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Li, N.; Liu, Y.; Su, H. Combination lung cancer chemotherapy: Design of a pH-sensitive transferrin-PEG-Hz-lipid conjugate for the co-delivery of docetaxel and baicalin. Biomed. Pharmacother. 2017, 95, 548–555. [Google Scholar] [CrossRef]
- Liang, Y.; Tian, B.; Zhang, J.; Li, K.; Wang, L.; Han, J.; Wu, Z. Tumor-targeted polymeric nanostructured lipid carriers with precise ratiometric control over dual-drug loading for combination therapy in non-small-cell lung cancer. Int. J. Nanomed. 2017, 12, 1699–1715. [Google Scholar] [CrossRef]
- Cao, X.; Luo, J.; Gong, T.; Zhang, Z.-R.; Sun, X.; Fu, Y. Coencapsulated doxorubicin and bromotetrandrine lipid nanoemulsions in reversing multidrug resistance in breast cancer in vitro and in vivo. Mol. Pharm. 2015, 12, 274–286. [Google Scholar] [CrossRef]
- Rocca, A.; Cecconetto, L.; Passardi, A.; Melegari, E.; Andreis, D.; Monti, M.; Maltoni, R.; Sarti, S.; Pietri, E.; Schirone, A.; et al. Phase Ib dose-finding trial of lapatinib plus pegylated liposomal doxorubicin in advanced HER2-positive breast cancer. Cancer Chemother. Pharmacol. 2017, 79, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Li, W.; Zhang, Y.; Zhang, B. Anti-tumor efficiency of paclitaxel and DNA when co-delivered by pH responsive ligand modified nanocarriers for breast cancer treatment. Biomed. Pharmacother. 2016, 83, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.K.; Singh, B.; Jain, A.; Nirbhavane, P.; Sharma, R.; Tyagi, R.K.; Kushwah, V.; Jain, S.; Katare, O.P. Fucose decorated solid-lipid nanocarriers mediate efficient delivery of methotrexate in breast cancer therapeutics. Coll. Surf. B Biointerfaces 2016, 146, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jia, X.; Niu, H. Nanostructured lipid carriers co-delivering lapachone and doxorubicin for overcoming multidrug resistance in breast cancer therapy. Int. J. Nanomed. 2018, 13, 4107–4119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, C.-C.; Chan, W.-K.-N.; Liu, K.-L.; Yang, Z.-J.; Zhang, H.-Q. Augmented Anticancer Effects of Cantharidin with Liposomal Encapsulation:In Vitro and In Vivo Evaluation. Molecules 2017, 22, 1052. [Google Scholar]
- Chang, M.; Wu, M.; Li, H. Antitumor activities of novel glycyrrhetinic acid-modified curcumin-loaded cationic liposomes in vitro and in H22 tumor-bearing mice. Drug Deliv. 2018, 25, 1984–1995. [Google Scholar] [CrossRef]
- Beg, M.S.; Brenner, A.J.; Sachdev, J.; Borad, M.; Kang, Y.-K.; Stoudemire, J.; Smith, S.; Bader, A.G.; Kim, S.; Hong, D.S. Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Investig. New Drugs 2017, 35, 180–188. [Google Scholar] [CrossRef]
- Grillone, A.; Riva, E.R.; Mondini, A.; Forte, C.; Calucci, L.; Innocenti, C.; de Julian Fernandez, C.; Cappello, V.; Gemmi, M.; Moscato, S.; et al. Active Targeting of Sorafenib: Preparation, Characterization, and In Vitro Testing of Drug-Loaded Magnetic Solid Lipid Nanoparticles. Adv. Healthc. Mater. 2015, 4, 1681–1690. [Google Scholar] [CrossRef]
- Harshita; Barkat, M.A.; Rizwanullah, M.; Beg, S.; Pottoo, F.H.; Siddiqui, S.; Ahmad, F.J. Paclitaxel-loaded Nanolipidic Carriers with Improved Oral Bioavailability and Anticancer Activity against Human Liver Carcinoma. AAPS PharmSciTech 2019, 20, 87. [Google Scholar]
- Ding, J.; Feng, M.; Wang, F.; Wang, H.; Guan, W. Targeting effect of PEGylated liposomes modified with the Arg-Gly-Asp sequence on gastric cancer. Oncol. Rep. 2015, 34, 1825–1834. [Google Scholar] [CrossRef]
- Yang, F.; Zheng, Z.; Zheng, L.; Qin, J.; Li, H.; Xue, X.; Gao, J.; Fang, G. SATB1 siRNA-encapsulated immunoliposomes conjugated with CD44 antibodies target and eliminate gastric cancer-initiating cells. OncoTargets Ther. 2018, 11, 6811–6825. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, R.; Sun, X.; Wang, T.; Liu, H.; Wang, S.-L. Intracellular uptake of etoposide-loaded solid lipid nanoparticles induces an enhancing inhibitory effect on gastric cancer through mitochondria-mediated apoptosis pathway. Int. J. Nanomed. 2014, 9, 3987–3998. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Y.; Meng, Y.-P.; Bo, L.-S.; Ke, W.-B. miR-542-3p Appended Sorafenib/All-trans Retinoic Acid (ATRA)-Loaded Lipid Nanoparticles to Enhance the Anticancer Efficacy in Gastric Cancers. Pharm. Res. 2017, 34, 2710–2719. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Geng, D.; Liu, H.; Li, Z.; Cao, J. Co-delivery of etoposide and curcumin by lipid nanoparticulate drug delivery system for the treatment of gastric tumors. Drug Deliv. 2016, 23, 3665–3673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu-Fayyad, A.; Nazzal, S. Gemcitabine-vitamin E conjugates: Synthesis, characterization, entrapment into nanoemulsions, and in-vitro deamination and antitumor activity. Int. J. Pharm. 2017, 528, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Schlesinger, M.; Rupp, A.; Schubert, R.; Nolting, J.; Wenzel, J.; Holdenrieder, S.; Brossart, P.; Bendas, G.; Feldmann, G. A liposomal formulation of the synthetic curcumin analog EF24 (Lipo-EF24) inhibits pancreatic cancer progression: Towards future combination therapies. J. Nanobiotechnol. 2016, 14, 57. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, Y.; Xia, D.; Guo, S.; Wang, F.; Zhang, X.; Gan, Y. Thermosensitive Liposomal Codelivery of HSA-Paclitaxel and HSA-Ellagic Acid Complexes for Enhanced Drug Perfusion and Efficacy Against Pancreatic Cancer. ACS Appl. Mater. Interfaces 2017, 9, 25138–25151. [Google Scholar] [CrossRef]
- Wang-Gillam, A.; Li, C.-P.; Bodoky, G.; Dean, A.; Shan, Y.-S.; Jameson, G.; Macarulla, T.; Lee, K.-H.; Cunningham, D.; Blanc, J.F.; et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): A global, randomised, open-label, phase 3 trial. Lancet 2016, 387, 545–557. [Google Scholar] [CrossRef]
- Moghimipour, E.; Rezaei, M.; Ramezani, Z.; Kouchak, M.; Amini, M.; Angali, K.A.; Dorkoosh, F.A.; Handali, S. Folic acid-modified liposomal drug delivery strategy for tumor targeting of 5-fluorouracil. Eur. J. Pharm. Sci. 2018, 114, 166–174. [Google Scholar] [CrossRef]
- Serini, S.; Cassano, R.; Corsetto, P.A.; Rizzo, A.M.; Calviello, G.; Trombino, S. Omega-3 PUFA Loaded in Resveratrol-Based Solid Lipid Nanoparticles: Physicochemical Properties and Antineoplastic Activities in Human Colorectal Cancer Cells In Vitro. Int. J. Mol. Sci. 2018, 19, 586. [Google Scholar] [CrossRef]
- Shen, M.Y.; Liu, T.-I.; Yu, T.-W.; Kv, R.; Chiang, W.-H.; Tsai, Y.-C.; Chen, H.-H.; Lin, S.-C.; Chiu, H.-C. Hierarchically targetable polysaccharide-coated solid lipid nanoparticles as an oral chemo/thermotherapy delivery system for local treatment of colon cancer. Biomaterials 2019, 197, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Negi, L.M.; Talegaonkar, S.; Jaggi, M.; Verma, A.K.; Verma, R.; Dobhal, S.; Kumar, V. Surface engineered nanostructured lipid carriers for targeting MDR tumor: Part I. Synthesis, characterization and in vitro investigation. Coll. Surf. B Biointerfaces 2014, 123, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, G.; El Sadda, R.R.; Botchkina, G.; Ojima, I.; Egan, J.; Amiji, M. Nanoemulsion formulation of a novel taxoid DHA-SBT-1214 inhibits prostate cancer stem cell-induced tumor growth. Cancer Lett. 2017, 406, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.J.; Chen, B.H. Preparation of catechin extracts and nanoemulsions from green tea leaf waste and their inhibition effect on prostate cancer cell PC-3. Int. J. Nanomed. 2016, 11, 1907–1926. [Google Scholar]
- Nassir, A.M.; Ibrahim, I.A.A.; Md, S.; Waris, M.; Tanuja Ain, M.R.; Ahmad, I.; Shahzad, N. Surface functionalized folate targeted oleuropein nano-liposomes for prostate tumor targeting: Invitro and invivo activity. Life Sci. 2019, 220, 136–146. [Google Scholar] [CrossRef]
- Hua, H.; Zhang, N.; Liu, D.; Song, L.; Liu, T.; Li, S.; Zhao, Y. Multifunctional gold nanorods and docetaxel-encapsulated liposomes for combined thermo- and chemotherapy. Int. J. Nanomed. 2017, 12, 7869–7884. [Google Scholar] [CrossRef]
- Abdulbaqi, I.M.; Assi, R.; Yaghmur, A.; Darwis, Y.; Mohtar, N.; Parumasivam, T.; Saqallah, F.; Wahab, H. Pulmonary Delivery of Anticancer Drugs via Lipid-Based Nanocarriers for the Treatment of Lung Cancer: An Update. Pharmaceuticals 2021, 14, 725. [Google Scholar] [CrossRef]
- Hegde, M.M.; Prabhu, S.; Mutalik, S.; Chatterjee, A.; Goda, J.S.; Rao, B.S.S. Multifunctional lipidic nanocarriers for effective therapy of glioblastoma: Recent advances in stimuli-responsive, receptor and subcellular targeted approaches. J. Pharm. Investig. 2021, 52, 49–74. [Google Scholar] [CrossRef]
- Laquintana, V.; Trapani, A.; Denora, N.; Wang, F.; Gallo, J.M.; Trapani, G. New strategies to deliver anticancer drugs to brain tumors. Expert Opin. Drug Deliv. 2009, 6, 1017–1032. [Google Scholar] [CrossRef]
- Kushwah, V.; Agrawal, A.K.; Dora, C.P.; Mallinson, D.; Lamprou, D.A.; Gupta, R.C.; Jain, S. Novel Gemcitabine Conjugated Albumin Nanoparticles: A Potential Strategy to Enhance Drug Efficacy in Pancreatic Cancer Treatment. Pharm. Res. 2017, 34, 2295–2311. [Google Scholar] [CrossRef]
- Ying, K.; Bai, B.; Gao, X.; Xu, Y.; Wang, H.; Xie, B. Orally Administrable Therapeutic Nanoparticles for the Treatment of Colorectal Cancer. Front. Bioeng. Biotechnol. 2021, 9, 670124. [Google Scholar] [CrossRef] [PubMed]
- Bottger, R.; Pauli, G.; Chao, P.-H.; AL Fayez, N.; Hohenwarter, L.; Li, S.-D. Lipid-based nanoparticle technologies for liver targeting. Adv. Drug Deliv. Rev. 2020, 154–155, 79–101. [Google Scholar] [CrossRef]
- Das, M.; Jain, R.; Agrawal, A.; Thanki, K.; Jain, S. Macromolecular bipill of gemcitabine and methotrexate facilitates tumor-specific dual drug therapy with higher benefit-to-risk ratio. Bioconjugate Chem. 2014, 25, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Yildirimer, L.; Thanh, N.T.; Loizidou, M.; Seifalian, A.M. Toxicology and clinical potential of nanoparticles. Nano Today 2011, 6, 585–607. [Google Scholar] [CrossRef] [PubMed]
- Azarnezhad, A.; Samadian, H.; Jaymand, M.; Sobhani, M.; Ahmadi, A. Toxicological profile of lipid-based nanostructures: Are they considered as completely safe nanocarriers? Crit. Rev. Toxicol. 2020, 50, 148–176. [Google Scholar] [CrossRef]







| Strategy | Mechanisms | Ref. |
|---|---|---|
| Enhanced solubilization | The lipids present in the gastrointestinal tract (GIT) increase the excretion of cholesterol and phospholipids (endogenous bile lipids), which further mediates the emulsification of the lipids present within the carrier system and solubilizes the drug. | [30] |
| Alteration in the biochemical barrier | Certain lipids and surfactants can decrease the intestinal secretions of the gastrointestinal wall, and prevent the metabolic activity of the enterocytes and lumen by alternating the P-glycoprotein, cytochromes, etc., thereby increasing the absorption of the drugs that are considered substrates of the stated efflux pump, and enzymes. | [25] |
| Alteration in the physical barrier | Some lipids and surfactants can promote intestinal absorption and membrane permeability by fluidizing the intestinal cell membrane and breaching the tight junctions. | [31] |
| Facilitation of lymphatic transport system | Lipids such as LCT (long-chain triglycerides) facilitate lipoprotein formation, which further facilitates their lymphatic transport. Hence, it could be stated that LNPs composed of LCT mediate lymphatic transport of poorly aqueous soluble drugs, thus bypassing the first-pass metabolism. | [25] |
| LNPs | Composition | Features | Advantages | Disadvantages | Status | Refs. |
|---|---|---|---|---|---|---|
| Liposomes | Phospholipids and cholesterol | Forms 1–20 phospholipid bilayers (vesicles) with globule size 30 nm to 3000 nm. Encapsulate hydrophilic and hydrophobic drugs. | Induce a controlled release profile. Enhances solubility of hydrophobic drugs, thereby increasing bioavailability | The structure is rigid. Controlled conditions are employed for reproducibility. Stability problems | Some are commercialized, while some are under clinical trials. | [40] |
| Nanoemulsions (NEs) | Oils, surfactants, and co-surfactants | Kinetically stable o/w dispersions. Have high surface area with small size (50–500 nm). Encapsulate both lipophilic and lipophobic drugs. | Form spontaneously. Increased reproducibility | Require high concentration of surfactant, hence can lead to toxicity. Scare choices of surfactants, as the surfactant used must be GRAS recommended. | Some are commercialized, while some are under clinical trials. | [41] |
| Solid-lipid nanoparticles (SLNs) | Solid lipids (fats), surfactants | Solid lipids instead of oil improve the lipidic core and provide stability and mobility to the drug within the lipidic core. | Exhibits delayed degradation of lipidic matrices allowing controlled release of the drug. | Exhibit reduced drug loading due to crystalline structure of the lipidic matrix, facilitating drug expulsion. Chances of agglomeration and polymorphic transitions. | Pre-clinical | [42] |
| Nanostructured lipid carriers (NLCs) | Solid lipids (fats), liquid lipids (fats or oils), and surfactants | NLC has a distorted structure which makes the matrix structure imperfect and creates spaces for the accommodation of active compounds. | Increased entrapment efficiency, with reduced drug leaking on storage. | Optimization required of the binary mixture, i.e., the ratio of solid and liquid lipids, otherwise, it would lead to cytotoxicity associated with the nature and concentration of lipid matrix. | Pre-clinical | [43] |
| Lipid–polymer hybrid nanoparticles (LPH-NPs) | Polymers, lipids | Hybrid vesicular structures integrate advantageous characteristics of polymers and liposomes in a single moiety. | Load efficiently one or multiple drugs with different properties. | - | Pre-clinical | [44] |
| Exosomes (Exo) | Cholesterol, diacylglycerol, surface proteins, heat shock proteins, lysosomal proteins, nucleic acids | Homogenous nanosized vesicles with size ranges from 30–150 nm. Formed by multivesicular bodies (MVB) after fusing with plasma membrane. | Immunocompatible | Rapid clearance from circulation after in vivo administration. No current manufacturing method. | Pre-clinical | [45,46] |
| Excipients | Results | Ref. |
|---|---|---|
| Liposomes | ||
| 1,2-dioleoyl-snglycero- 3-phosphocholine (DOPC) 1,2-distearoyl-sn-glycero- 3-phosphoethanolamine-N-[carboxy (polyethylene glycol)-2000] (DSPE-PEG-COOH) |
| [52] |
| DSPE-PEG2000 |
| [53] |
| Dioleoylphosphatidylethanolamine (DOPE) |
| [54] |
| Dipalmitoylphosphatidylcholine (DPPC) Distearoylphosphatidylcholine (DSPC) Cholesterol Distearoylphosphatidylethanolamine (DSPE) |
| [55] |
| LecithinCholesterol |
| [56] |
| Nanoemulsion (NEs) | ||
| Cod liver oil Lysophophatidylcholine (LPC), lysophophatidic acid (LPA), DSPE-PEG (2000) |
| [62] |
| Soya lecithin Kolliphor® HS15 |
| [63] |
| Soybean phospholipids Cholesterol |
| [64] |
| Miglyol 812 Phosphatidylcholine |
| [65] |
| Solid lipid nanoparticles (SLNs) | ||
| GMS (Glyceryl monostearate) Tween 80 |
| [75] |
| Palmitic acid Pluronic F-68 Soy lecithin |
| [76] |
| GMS SA (Stearic acid) Compritol ATO 888 Tween 80 as a surfactant |
| [77] |
| Stearyl amine Tween 80, Pluronic F-68 |
| [78] |
| [79] | |
| Nanostructured lipid carriers (NLCs) | ||
| Compritol ATO 888 Medium chain triglycerides (MCT) Tween 80 Soya lecithin |
| [81] |
| Precirol ATO 5 Maisine 35-1 Cremophor RH40 |
| [82] |
| Compritol 888 ATO Docosahexaenoic acid (DHA) Tween 80 |
| [83] |
| GMS Caproyl 90 Labrasol |
| [84] |
| GMS Caproyl 90 Poloxamer 188 |
| [85] |
| Lipid–Polymer hybrid nanoparticles (LPH-NPs) | ||
| HPESO (hydrolyzed polymer of epoxidized soyabean oil) Myristic acid |
| [87] |
| Poly lactide glycolic acid (PLGA) Polyethylene glycol (PEG) Dioleoylphosphatidic acid (DOPA) |
| [88] |
| Gelucire 48/16, Phospholipid 90NG Phospholipid S100 |
| [89] |
| PLGA, DSPE-PEG Lecithin |
| [90] |
| Exosomes (Exo) | ||
| Mesenchymal stem cells (MSCs), Surface proteins: tetraspanins (CD63, CD9, CD81), heat shock proteins (Hsc70), lysosomal proteins (Lamp2b), and fusion proteins (flotillin, annexin). |
| [101] |
| Human monocyte-derived macrophage cells Surface proteins (Exosomal marker proteins): CD81 and CD63. |
| [102] |
| Human fetal lung fibroblast Surface proteins (Exosomal markers): TSG101 and CD81 |
| [103] |
| Macrophage Surface proteins |
| [104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaudhuri, A.; Kumar, D.N.; Shaik, R.A.; Eid, B.G.; Abdel-Naim, A.B.; Md, S.; Ahmad, A.; Agrawal, A.K. Lipid-Based Nanoparticles as a Pivotal Delivery Approach in Triple Negative Breast Cancer (TNBC) Therapy. Int. J. Mol. Sci. 2022, 23, 10068. https://doi.org/10.3390/ijms231710068
Chaudhuri A, Kumar DN, Shaik RA, Eid BG, Abdel-Naim AB, Md S, Ahmad A, Agrawal AK. Lipid-Based Nanoparticles as a Pivotal Delivery Approach in Triple Negative Breast Cancer (TNBC) Therapy. International Journal of Molecular Sciences. 2022; 23(17):10068. https://doi.org/10.3390/ijms231710068
Chicago/Turabian StyleChaudhuri, Aiswarya, Dulla Naveen Kumar, Rasheed A. Shaik, Basma G. Eid, Ashraf B. Abdel-Naim, Shadab Md, Aftab Ahmad, and Ashish Kumar Agrawal. 2022. "Lipid-Based Nanoparticles as a Pivotal Delivery Approach in Triple Negative Breast Cancer (TNBC) Therapy" International Journal of Molecular Sciences 23, no. 17: 10068. https://doi.org/10.3390/ijms231710068