Albumin/Thiacalix[4]arene Nanoparticles as Potential Therapeutic Systems: Role of the Macrocycle for Stabilization of Monomeric Protein and Self-Assembly with Ciprofloxacin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Tetrasubstituted p-tert-butylthiacalix[4]arene Containing Sulfobetaine Fragments
2.2. Cytotoxicity of Test Compounds 3, 4 on Cancer and Non-Cancer Human Cell Lines
2.3. Association of Water-Soluble Sulfobetaines 3, 4 with BSA in Solution and Solid Phase
2.4. UV-Vis and Fluorescence Spectroscopy of Water-Soluble Sulfobetaines 3 and 4 with BSA
2.5. Circular Dichroism Spectroscopy of Water-Soluble Sulfobetaines 3 and 4 with BSA and Molecular Docking of Their Associates
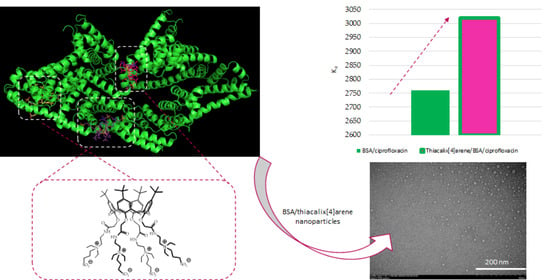
2.6. Effect of Water-Soluble Sulfobetaines 3 and 4 on the Efficiency BSA/Ciprofloxacin Interactions
3. Materials and Methods
3.1. General
- General procedure for the synthesis of compounds 3 and 4
3.2. UV-Visible Spectroscopy
3.3. Dynamic Light Scattering (DLS)
3.3.1. Particles’ Size
3.3.2. Zeta Potentials
3.4. Transmission Electron Microscopy (TEM)
3.5. Fluorescence Spectroscopy
3.6. Circular Dichroism (CD) Studies
3.7. Cytotoxicity of Test Compounds on Cancer and Normal Human Cell Lines
3.8. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chruszcz, M.; Mikolajczak, K.; Mank, N.; Majorek, K.A.; Porebski, P.J.; Minor, W. Serum albumins—Unusual allergens. Biochim. Biophys. Acta-Gen. Subj. 2013, 1830, 5375–5381. [Google Scholar] [CrossRef] [PubMed]
- Spada, A.; Emami, J.; Tuszynski, J.A.; Lavasanifar, A. The uniqueness of albumin as a carrier in nanodrug delivery. Mol. Pharm. 2021, 18, 1862–1894. [Google Scholar] [CrossRef] [PubMed]
- Perrudet-Badoux, A.; Frei, P.C. Immunogenicity of Bovine Serum Albumin in Adult Rabbits of Various Strains. Int. Arch. Allergy Immunol. 1971, 40, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Yurina, E.S.; Gubarev, Y.A.; Lebedeva, N.S. A study of protein aggregation activators in molecular complexes of cationic porphyrins and chlorin with BSA. J. Mol. Liq. 2021, 338, 116632. [Google Scholar] [CrossRef]
- Holm, N.K.; Jespersen, S.K.; Thomassen, L.V.; Wolff, T.Y.; Sehgal, P.; Thomsen, L.A.; Christiansen, G.; Andersen, C.B.; Knudsen, A.D.; Otzen, D.E. Aggregation and fibrillation of bovine serum albumin. Biochim. Biophys. Acta-Proteins Proteom. 2007, 1774, 1128–1138. [Google Scholar] [CrossRef]
- Bulone, D.; Martorana, V.; Biagio, P.L.S. Effects of intermediates on aggregation of native bovine serum albumin. Biophys. Chem. 2001, 91, 61–69. [Google Scholar] [CrossRef]
- Militello, V.; Casarino, C.; Emanuele, A.; Giostra, A.; Pullara, F.; Leone, M. Aggregation kinetics of bovine serum albumin studied by FTIR spectroscopy and light scattering. Biophys. Chem. 2004, 107, 175–187. [Google Scholar] [CrossRef]
- Yohannes, G.; Wiedmer, S.K.; Elomaa, M.; Jussila, M.; Aseyev, V.; Riekkola, M.-L. Thermal aggregation of bovine serum albumin studied by asymmetrical flow field-flow fractionation. Anal. Chim. Acta 2010, 675, 191–198. [Google Scholar] [CrossRef]
- Xie, B.; Li, X.; Dong, X.-Y.; Sun, Y. Insight into the Inhibition Effect of Acidulated Serum Albumin on Amyloid β-Protein Fibrillogenesis and Cytotoxicity. Langmuir 2014, 30, 9789–9796. [Google Scholar] [CrossRef]
- Nag, R.; Rao, C.P. Calixarene-mediated host–guest interactions leading to supramolecular assemblies: Visualization by microscopy. Chem. Commun. 2022, 58, 6044–6063. [Google Scholar] [CrossRef]
- Engilberge, S.; Rennie, M.L.; Dumont, E.; Crowley, P.B. Tuning protein frameworks via auxiliary supramolecular interactions. ACS Nano 2019, 13, 10343–10350. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.; Zhao, X.; Xu, L.; Zheng, Y.; Li, H.B.; Guo, D.S.; Linqi, S.; Liu, Y. Calixarene-modified albumin for stoichiometric delivery of multiple drugs in combination-chemotherapy. Theranostics 2022, 12, 3747. [Google Scholar] [CrossRef] [PubMed]
- Quaglio, D.; Polli, F.; Del Plato, C.; Cianfoni, G.; Tortora, C.; Mazzei, F.; Botta, B.; Calcaterra, A.; Ghirga, F. Calixarene: A versatile scaffold for the development of highly sensitive biosensors. Supramol. Chem. 2021, 33, 345–369. [Google Scholar] [CrossRef]
- Crowley, P.B. Protein–Calixarene Complexation: From Recognition to Assembly. Acc. Chem. Res. 2022, 55, 2019–2032. [Google Scholar] [CrossRef]
- Morohashi, N.; Narumi, F.; Iki, N.; Hattori, T.; Miyano, S. Thiacalixarenes. Chem. Rev. 2006, 106, 5291–5316. [Google Scholar] [CrossRef]
- Vavilova, A.A.; Nosov, R.V.; Yakimova, L.S.; Antipin, I.S.; Stoikov, I.I. Synthesis of Photo-Switchable Derivatives of p-tert-Butyl Thiacalix[4]arenes Containing Ethoxycarbonyl and 4-Amidoazobenzene Fragments in the Lower Rim Substituents. Macroheterocycles 2013, 6, 219–226. [Google Scholar] [CrossRef]
- Puplampu, J.B.; Yakimova, L.S.; Vavilova, A.A.; Fayzullin, D.A.; Zuev, Y.F.; Stoikov, I.I. Synthesis of p-tert-butylthiacalix[4]arenes functionalized with tris(2-aminoethyl)amine fragments at the lower rim and their interaction with model lipid membranes. Macroheterocycles 2014, 7, 337–344. [Google Scholar] [CrossRef]
- Xu, Z.; Jia, S.; Wang, W.; Yuan, Z.; Ravoo, B.J.; Guo, D.-S. Heteromultivalent peptide recognition by co-assembly of cyclodextrin and calixarene amphiphiles enables inhibition of amyloid fibrillation. Nat. Chem. 2019, 11, 86–93. [Google Scholar] [CrossRef]
- Tian, H.-W.; Liu, Y.-C.; Guo, D.-S. Assembling features of calixarene-based amphiphiles and supra-amphiphiles. Mater. Chem. Front. 2020, 4, 46–98. [Google Scholar] [CrossRef]
- Yakimova, L.S.; Gilmanova, L.H.; Evtugyn, V.G.; Osin, Y.N.; Stoikov, I.I. Self-assembled fractal hybrid dendrites from water-soluble anionic (thia)calix[4]arenes and Ag+. J. Nanopart. Res. 2017, 19, 173–183. [Google Scholar] [CrossRef]
- Yakimova, L.; Padnya, P.; Tereshina, D.; Kunafina, A.; Nugmanova, A.; Osin, Y.; Evtugyn, V.; Stoikov, I. Interpolyelectrolyte mixed nanoparticles from anionic and cationic thiacalix[4]arenes for selective recognition of model biopolymers. J. Mol. Liq. 2019, 279, 9–17. [Google Scholar] [CrossRef]
- Kashapov, R.; Razuvayeva, Y.; Ziganshina, A.; Sergeeva, T.; Lukashenko, S.; Sapunova, A.; Voloshina, A.; Kashapova, N.; Nizameev, I.; Salnikov, V.; et al. Supraamphiphilic systems based on metallosurfactant and calix[4]resorcinol: Self-assembly and drug delivery potential. Inorg. Chem. 2020, 59, 18276–18286. [Google Scholar] [CrossRef]
- Yakimova, L.; Vavilova, A.; Shibaeva, K.; Sultanaev, V.; Mukhametzyanov, T.; Stoikov, I. Supramolecular approaches to the formation of nanostructures based on phosphonate-thiacalix[4]arenes, their selective lysozyme recognition. Colloid Surface Asp. 2021, 611, 125897. [Google Scholar] [CrossRef]
- Mostovaya, O.A.; Valiullina, Y.A.; Chan, C.T.; Potrekeeva, O.S.; Padnya, P.L.; Zuev, Y.F.; Stoikov, I.I. Synthetic modulator of chymotrypsin activity based on p-tert-butylthiacalix[4] arene. Mendeleev Commun. 2019, 29, 520–522. [Google Scholar] [CrossRef]
- Yakimova, L.; Kunafina, A.; Nugmanova, A.; Padnya, P.; Voloshina, A.; Petrov, K.; & Stoikov, I. Structure–Activity Relationship of the Thiacalix[4]arenes Family with Sulfobetaine Fragments: Self-Assembly and Cytotoxic Effect against Cancer Cell Lines. Molecules 2022, 27, 1364. [Google Scholar] [CrossRef]
- Ghosh, S.; Moulik, S.P. Interfacial and micellization behaviors of binary and ternary mixtures of amphiphiles (Tween-20, Brij-35, and sodium dodecyl sulfate) in aqueous medium. J. Colloid Interface Sci. 1998, 208, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Schönemann, E.; Koc, J.; Aldred, N.; Clare, A.S.; Laschewsky, A.; Rosenhahn, A.; Wischerhoff, E. Synthesis of novel sulfobetaine polymers with differing dipole orientations in their side chains, and their effects on the antifouling properties. Macromol. Rapid Commun. 2022, 41, 1900447. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Zhu, Y.H.; Wang, X.Y.; Shen, C.; Wei, X.W.; Xu, T.; He, Z.Y. Novel zwitterionic vectors: Multi-functional delivery systems for therapeutic genes and drugs. Comput. Struct. Biotechnol. J. 2020, 18, 1980–1999. [Google Scholar] [CrossRef]
- Terp, D.K.; Rybak, M.J. Ciprofloxacin. Drug Intell. Clin. Pharm. 1987, 21, 568–574. [Google Scholar] [CrossRef]
- Suaifan, G.A.R.Y.; Mohammed, A.A.M.; Alkhawaja, B.A. Fluoroquinolones’ Biological Activities against Laboratory Microbes and Cancer Cell Lines. Molecules 2022, 27, 1658. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Blanco-Fernandez, B.; Puga, A.M.; Concheiro, A. Crosslinked ionic polysaccharides for stimuli-sensitive drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 1148–1171. [Google Scholar] [CrossRef] [PubMed]
- Măciucă, A.-M.; Munteanu, A.-C.; Uivarosi, V. Quinolone Complexes with Lanthanide Ions: An Insight into their Analytical Applications and Biological Activity. Molecules 2020, 25, 1347. [Google Scholar] [CrossRef] [PubMed]
- Yakimova, L.S.; Padnya, P.L.; Kunafina, A.F.; Nugmanova, A.R.; & Stoikov, I.I. Sulfobetaine derivatives of thiacalix[4] arene: Synthesis and supramolecular self-assembly of submicron aggregates with AgI cations. Mendeleev Commun. 2019, 29, 86–88. [Google Scholar] [CrossRef]
- Andreyko, E.A.; Padnya, P.L.; Daminova, R.R.; Stoikov, I.I. Supramolecular “containers”: Self-assembly and functionalization of thiacalix[4] arenes for recognition of amino-and dicarboxylic acids. RSC Adv. 2014, 4, 3556–3565. [Google Scholar] [CrossRef]
- Padnya, P.L.; Bayarashov, E.E.; Potrekeeva, O.S.; Stoikov, I.I. Effect of the alkylidene spacer and the p-tert-butylthiacalix[4]arene macrocyclic platform on the reactivity of the hydroxyl groups in the acylation reaction. Russ. J. Gen. Chem. 2017, 87, 2111–2114. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Chawla, H.M.; Pant, N.; Pratap, S.; Upreti, S.S. Synthesis of conformationally diverse tetrathiacalix[4]arene(amido)crowns and tetrathiacalix[4]arene amides with pendant amine functions. Tetrahedron 2006, 62, 8974–8981. [Google Scholar] [CrossRef]
- Ayoup, M.S.; Wahby, Y.; Abdel-Hamid, H.; Ramadan, E.S.; Teleb, M.; Abu-Serie, M.M.; Noby, A. Design, synthesis and biological evaluation of novel α-acyloxy carboxamides via Passerini reaction as caspase 3/7 activators. Eur. J. Med. Chem. 2019, 168, 340–356. [Google Scholar] [CrossRef]
- Scanavachi, G.; Espinosa, Y.R.; Yoneda, J.S.; Rial, R.; Ruso, J.M.; Itri, R. Aggregation features of partially unfolded bovine serum albumin modulated by hydrogenated and fluorinated surfactants: Molecular dynamics insights and experimental approaches. J. Colloid Interface Sci. 2020, 572, 9–21. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential–what they are and what they are not? J Control Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Valstar, A.; Almgren, M.; Brown, W.; Vasilescu, M. The Interaction of Bovine Serum Albumin with Surfactants Studied by Light Scattering. Langmuir 2000, 16, 922–927. [Google Scholar] [CrossRef]
- Freifelder, D.M. Physical Biochemistry: Applications to Biochemistry and Molecular Biology (Life Sciences/Biochemistry), 2nd ed.; W. H. Freeman: San Francisco, CA, USA, 1982; 761p. [Google Scholar]
- Jahanban-Esfahlan, A.; Roufegarinejad, L.; Jahanban-Esfahlan, R.; Tabibiazar, M.; Amarowicz, R. Latest developments in the detection and separation of bovine serum albumin using molecularly imprinted polymers. Talanta 2020, 207, 120317. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2006; p. 954. [Google Scholar]
- Reed, R.G.; Putnam, F.W.; Peters, T., Jr. Sequence of residues 400–403 of bovine serum albumin. Biochem. J. 1980, 191, 867. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Garrido, A.; del Castillo-Santaella, T.; Yang, Y.; Galisteo-González, F.; José Gálvez-Ruiz, M.; Molina-Bolívar, J.A.; Holgado-Terriza, J.A.; Cabrerizo-Vílchez, M.Á.; Maldonado-Valderrama, J. Applications of serum albumins in delivery systems: Differences in interfacial behaviour and interacting abilities with polysaccharides. Adv. Colloid Interface Sci. 2021, 290, 102365. [Google Scholar] [CrossRef]
- Zhou, K.L.; Pan, D.Q.; Lou, Y.Y.; Shi, J.H. Intermolecular interaction of fosinopril with bovine serum albumin (BSA): The multi-spectroscopic and computational investigation. J. Mol. Recognit. 2018, 31, e2716. [Google Scholar] [CrossRef] [PubMed]
- Bhowal, A.C.; Kundu, S. A comparative study on intrinsic fluorescence of BSA and lysozyme proteins in presence of different divalent ions from their solution and thin film conformations. Luminescence 2018, 33, 267–276. [Google Scholar] [CrossRef]
- Mostovaya, O.A.; Gorbachuk, V.V.; Bazanova, O.B.; Gerasimov, A.V.; Evtugyn, V.G.; Osin, Y.N.; Myakushev, V.D.; Rizvanov, I.K.; Stoikov, I.I. Thiacalixarene “knot” effect on protein binding by oligolactic acid particles. Mater. Chem. Front. 2019, 3, 292–300. [Google Scholar] [CrossRef]
- Huang, F.; Pan, F.; Wang, L.; Xiao, Z.; He, J.; Yan, M.; Wang, J.; Qiu, W.; Liu, M.; Dong, H. The interaction between citronellol and bovine serum albumin: Spectroscopic, computational and thermal imaging studies. J. Mol. Struct. 2022, 1251, 131986. [Google Scholar] [CrossRef]
- Janek, T.; Czyżnikowska, Z.; Łuczyński, J.; Gudiña, E.J.; Rodrigues, L.R.; Gałęzowska, J. Physicochemical study of biomolecular interactions between lysosomotropic surfactants and bovine serum albumin. Colloids Surf. B 2017, 159, 750–758. [Google Scholar] [CrossRef]
- Khan, F.I.; Rehman, M.T.; Sameena, F.; Hussain, T.; Al Ajmi, M.F.; Lai, D.; Khan, M.K.A. Investigating the binding mechanism of topiramate with bovine serum albumin using spectroscopic and computational methods. J. Mol. Recognit. 2022, 35, e2958. [Google Scholar] [CrossRef]
- Liu, B.; Guo, Y.; Wang, J.; Xu, R.; Wang, X.; Wang, D.; Zhang, L.Q.; Xu, Y.N. Spectroscopic studies on the interaction and sonodynamic damage of neutral red (NR) to bovine serum albumin (BSA). J. Lumin. 2010, 130, 1036–1043. [Google Scholar] [CrossRef]
- Shi, J.H.; Chen, J.; Wang, J.; Zhu, Y.Y.; Wang, Q. Binding interaction of sorafenib with bovine serum albumin: Spectroscopic methodologies and molecular docking. Spectrochim. Acta A 2015, 149, 630–637. [Google Scholar] [CrossRef]
- Pabbathi, A.; Patra, S.; Samanta, A. Structural transformation of bovine serum albumin induced by dimethyl sulfoxide and probed by fluorescence correlation spectroscopy and additional methods. ChemPhysChem 2013, 14, 2441–2449. [Google Scholar] [CrossRef] [PubMed]
- van de Weert, M.; Hoechstetter, J.; Hennink, W.E.; Crommelin, D.J. The effect of a water/organic solvent interface on the structural stability of lysozyme. J. Control Release 2000, 68, 351–359. [Google Scholar] [CrossRef]
- Devaurs, D.; Antunes, D.A.; Hall-Swan, S.; Mitchell, N.; Moll, M.; Lizée, G.; Kavraki, L.E. Using parallelized incremental meta-docking can solve the conformational sampling issue when docking large ligands to proteins. BMC Mol. Biol. 2019, 20, 42. [Google Scholar] [CrossRef] [PubMed]
- Antunes, D.A.; Moll, M.; Devaurs, D.; Jackson, K.R.; Lizée, G.; Kavraki, L.E. DINC 2.0: A new protein-peptide docking webserver using an incremental approach. Cancer Res. 2017, 77, 55–57. [Google Scholar] [CrossRef]
- Thordarson, P. Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev. 2011, 40, 1305–1323. [Google Scholar] [CrossRef]
- Hibbert, D.B.; Thordarson, P. The death of the Job plot, transparency, open science and online tools, uncertainty estimation methods and other developments in supramolecular chemistry data analysis. Chem. Commun. 2016, 52, 12792–12805. [Google Scholar] [CrossRef]
- Iki, N.; Narumi, F.; Fujimoto, T.; Morohashi, N.; Miyano, S. Selective synthesis of three conformational isomers of tetrakis [(ethoxycarbonyl) methoxy] thiacalix[4] arene and their complexation properties towards alkali metal ions. J. Chem. Soc. Perkin Trans. 2 1998, 12, 2745–2750. [Google Scholar] [CrossRef]
- Padnya, P.L.; Andreyko, E.A.; Mostovaya, O.A.; Rizvanov, I.K.; Stoikov, I.I. The synthesis of new amphiphilic p-tert-butylthiacalix[4] arenes containing peptide fragments and their interaction with DNA. Org. Biomol. Chem. 2015, 13, 5894–5904. [Google Scholar] [CrossRef]







| Test Compounds | IC50 (µM) | |||
|---|---|---|---|---|
| M-HeLa | HuTu 80 | MCF-7 | Chang Liver | |
| 3 | 500 | 500 | 500 | 500 |
| 4 | >500 | >500 | >500 | >500 |
| System | C, M | ζ, mV | PDI |
|---|---|---|---|
| 3/BSA | 5 × 10−5/5 × 10−5 | −6.86 ± 0.92 | 0.15 ± 0.02 |
| 3/BSA | 1 × 10−5/1 × 10−5 | −4.91 ± 0.27 | 0.22 ± 0.04 |
| 4/BSA | 5 × 10−5/5 × 10−4 | −9.74 ± 1.71 | 0.22 ± 0.03 |
| 4/BSA | 1 × 10−5/1 × 10−4 | −9.24 ± 1.14 | 0.15 ± 0.02 |
| 4/BSA | 5 × 10−6/5 × 10−5 | −9.87 ± 2.62 | 0.15 ± 0.01 |
| System | T, K | lgKa | ΔH0, KJ mol−1 | ΔS0, J mol−1 R−1 | ΔG0, KJ mol−1 |
|---|---|---|---|---|---|
| 3/BSA | 278 | 3.72 | −40.9 | −76.9 | −19.5 |
| 293 | 3.16 | −18.0 | |||
| 308 | 2.98 | −17.2 | |||
| 4/BSA | 278 | 3.94 | −16.3 | 16.7 | −21.0 |
| 293 | 3.80 | −21.3 | |||
| 308 | 3.64 | −21.5 |
| Macrocycle | CBSA, μM | Macrocycle/BSA Molar Ratio | Ka [M−1] (% Error) |
|---|---|---|---|
| - | 10 | 0/1 | 2760 (3.7) |
| 3 | 10 | 1/1 | 3020 (2.5) |
| 100 | 1/10 | 2710 (2.2) | |
| 4 | 10 | 1/1 | 1220 (3.9) |
| 100 | 1/10 | 2010 (2.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakimova, L.; Kunafina, A.; Mostovaya, O.; Padnya, P.; Mukhametzyanov, T.; Voloshina, A.; Petrov, K.; Boldyrev, A.; Stoikov, I. Albumin/Thiacalix[4]arene Nanoparticles as Potential Therapeutic Systems: Role of the Macrocycle for Stabilization of Monomeric Protein and Self-Assembly with Ciprofloxacin. Int. J. Mol. Sci. 2022, 23, 10040. https://doi.org/10.3390/ijms231710040
Yakimova L, Kunafina A, Mostovaya O, Padnya P, Mukhametzyanov T, Voloshina A, Petrov K, Boldyrev A, Stoikov I. Albumin/Thiacalix[4]arene Nanoparticles as Potential Therapeutic Systems: Role of the Macrocycle for Stabilization of Monomeric Protein and Self-Assembly with Ciprofloxacin. International Journal of Molecular Sciences. 2022; 23(17):10040. https://doi.org/10.3390/ijms231710040
Chicago/Turabian StyleYakimova, Luidmila, Aisylu Kunafina, Olga Mostovaya, Pavel Padnya, Timur Mukhametzyanov, Alexandra Voloshina, Konstantin Petrov, Artur Boldyrev, and Ivan Stoikov. 2022. "Albumin/Thiacalix[4]arene Nanoparticles as Potential Therapeutic Systems: Role of the Macrocycle for Stabilization of Monomeric Protein and Self-Assembly with Ciprofloxacin" International Journal of Molecular Sciences 23, no. 17: 10040. https://doi.org/10.3390/ijms231710040