Preparation and Antibacterial Activity of Nano Copper Oxide- Loaded Zeolite 10X
Abstract
:1. Introduction
2. Results and Discussion
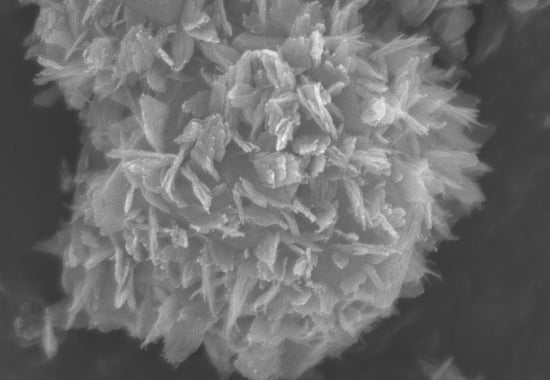
2.1. Characterization of Zeolite
2.2. Antibacterial Activity of CuO-Zeolite NCs
3. Materials and Methods
3.1. Materials
3.2. Preparation of CuO-Zeolite NCs
3.3. Characterization
3.4. Antibacterial Activity Assays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, I.; Islam, M.; Habis, N.A.; Parvez, S. Hot-pressed graphene nanoplatelets or/and zirconia reinforced hybrid alumina nanocomposites with improved toughness and mechanical characteristics. J. Mater. Sci. Technol. 2020, 40, 135–145. [Google Scholar] [CrossRef]
- Sultana, S.; Rafiuddin; Khan, M.Z.; Umar, K.; Muneer, M. Electrical, thermal, photocatalytic and antibacterial studies of metallic oxide nanocomposite doped polyaniline. J. Mater. Sci. Technol. 2013, 29, 795–800. [Google Scholar]
- Manickam-Periyaraman, P.; Espinosa, J.C.; Ferrer, B.; Subramanian, S.; Álvaro, M.; García, H.; Navalón, S. Bimetallic iron-copper oxide nanoparticles supported on nanometric diamond as efficient and stable sunlight-assisted Fenton photocatalyst. Chem. Eng. J. 2020, 393, 124770. [Google Scholar] [CrossRef]
- Zhang, D.Z.; Wu, J.F.; Cao, Y.H. Ultrasensitive H2S gas detection at room temperature based on copperoxide/molybdenum disulfide nanocomposite with synergistic effect. Sens. Actuators B-Chem. 2019, 287, 346–355. [Google Scholar] [CrossRef]
- Yang, Z.; Yi, C.; Lv, S.J.; Sheng, Y.H.; Wen, W.; Zhang, X.H.; Wang, S.F. Development of a lateral flow strip biosensor based on copper oxide nanoparticles for rapid and sensitive detection of HPV16 DNA. Sens. Actuators B-Chem. 2019, 285, 326–332. [Google Scholar] [CrossRef]
- Gao, H.T.; Mao, F.; Song, Y.C.; Hong, J.J.; Yan, Y.Y. Effect of adding copper oxide nanoparticles on the mass/heat transfer in falling film absorption. Appl. Therm. Eng. 2020, 181, 115937. [Google Scholar] [CrossRef]
- Siddiqui, H.; Parra, M.R.; Pandey, P.; Qureshi, M.S.; Haque, F.Z. Utility of copper oxide nanoparticles (CuO-NPs) as efficient electron donor material in bulk-heterojunction solar cells with enhanced power conversion efficiency. J. Sci. Adv. Mater. Dev. 2020, 5, 104–110. [Google Scholar] [CrossRef]
- Akintelu, S.A.; Folorunso, A.S.; Folorunso, F.A.; Oyebamiji, A.K. Green synthesis of copper oxide nanoparticles for biomedical applicationand environmental remediation. Heliyon 2020, 6, e04508. [Google Scholar] [CrossRef]
- Hassabo, A.G.; El-Naggar, M.E.; Mohamed, A.L.; Hebeish, A.A. Development of multifunctional modified cotton fabric with tri-component nanoparticles of silver, copper and zinc oxide. Carbohyd. Polym. 2019, 210, 144–156. [Google Scholar] [CrossRef]
- Ananth, A.; Dharaneedharan, S.; Heo, M.S.; Mok, Y.S. Copper oxide nanomaterials: Synthesis, characterization and structure-specific antibacterial performance. Chem. Eng. J. 2015, 262, 179–188. [Google Scholar] [CrossRef]
- Perla, V.K.; Ghosh, S.K.; Mallick, K. Bipolar resistive switching behavior of carbon nitride supported copper oxide nanoparticles. Chem. Phys. Lett. 2020, 754, 137650. [Google Scholar] [CrossRef]
- Murugappan, G.; Sreeram, K.J. Nano-biocatalyst: Bi-functionalization of protease and amylase on copper oxide nanoparticles. Colloids Surf. B. 2021, 197, 111386. [Google Scholar] [CrossRef]
- Rajabzadeh, M.; Khalifeh, R.; Eshghi, H.; Hafizi, A. Design and synthesis of CuO@SiO2 multi-yolk@shell and its application as a new catalyst for CO2 fixation reaction under solventless condition. J. Ind. Eng. Chem. 2020, 89, 458–469. [Google Scholar] [CrossRef]
- Tommalieh, M.J. Gamma radiation assisted modification on electrical properties of polyvinyl pyrrolidone/polyethylene oxide blend doped by copper oxide nanoparticles. Radiat. Phys. Chem. 2021, 179, 109236. [Google Scholar] [CrossRef]
- Noroozi, R.; Al-Musawi, T.J.; Kazemian, H.; Kalhori, E.M.; Zarrabi, M. Removal of cyanide using surface-modified Linde Type-A zeolite nanoparticles as an efficient and eco-friendly material. J. Water Process Eng. 2018, 21, 44–51. [Google Scholar] [CrossRef]
- Araki, S.; Li, T.; Li, K.; Yamamoto, H. Preparation of zeolite hollow fibers for high-efficiency cadmium removal from waste water. Sep. Purif. Technol. 2019, 221, 393–398. [Google Scholar] [CrossRef]
- Meng, T.; Ren, N.; Ma, Z. Silicalite-1@Cu-ZSM-5 core-shell catalyst for N2O decomposition. J. Mol. Catal. A-Chem. 2015, 404–405, 233–239. [Google Scholar] [CrossRef]
- Soori, F.; Nezamzadeh-Ejhieh, A. Synergistic effects of copper oxide-zeolite nanoparticles composite on photocatalytic degradation of 2,6-dimethylphenol aqueous solution. J. Mol. Liq. 2018, 255, 250–256. [Google Scholar] [CrossRef]
- Cheng, M.; Wang, Y.; Wang, W.H.G.; Wang, W.; Zhu, X.L.; Li, C.Y. Promoting effect of copper oxide on CsX zeolite catalyst for side-chain alkylation of toluene with methanol. Micropor. Mesopor. Mat. 2021, 311, 110732. [Google Scholar] [CrossRef]
- Javadhesari, S.M.; Alipour, S.; Mohammadnejad, S.; Akbarpour, M.R. Antibacterial activity of ultra-small copper oxide (II) nanoparticles synthesized by mechanochemical processing against S. aureus and E. coli. Mater. Sci. Eng. C-Mater. 2019, 105, 110011. [Google Scholar] [CrossRef]
- Nabila, M.I.; Kannabiran, K. Biosynthesis, characterization and antibacterial activity of copper oxide nanoparticles (CuO NPs) from actinomycetes. Biocatal. Agric. Biotechnol. 2018, 15, 56–62. [Google Scholar] [CrossRef]
- Alswat, A.A.; Ahmad, M.B.; Hussein, M.Z.; Ibrahim, N.A.; Saleh, T.A. Copper oxide nanoparticles-loaded zeolite and its characteristics and antibacterial activities. J. Mater. Sci. Technol. 2017, 3, 889–896. [Google Scholar] [CrossRef]
- Ninan, N.; Muthiah, M.; Yahaya, N.A.B.; Park, I.K.; Elain, A.; Wong, T.W.; Thomas, S.; Grohens, Y. Antibacterial and wound healing analysis of gelatin/zeolite scaffolds. Colloids Surf. B 2014, 115, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, H.; Higuchi, T.; Jayakumar, R.; Furuike, T.; Tamura, H. XRD studies of β-chitin from squid pen with calcium solvent. Int. J. Biol. Macromol. 2008, 42, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Brazlauskas, M.; Kitrys, S. Synthesis and properties of CuO/zeolite sandwich type adsorbent-catalysts. Chin. J. Catal. 2008, 29, 25–30. [Google Scholar] [CrossRef]
- Alswat, A.A.; Ahmad, M.B.; Saleh, T.A. Preparation and characterization of zeolite\zinc oxide-copper oxide nanocomposite: Antibacterial activities. Colloid Interface Sci. 2017, 16, 19–24. [Google Scholar] [CrossRef]
- Pandiyarajan, T.; Udayabhaskar, R.; Vignesh, S.; James, R.A.; Karthikeyan, B. Synthesis and concentration dependent antibacterial activities of CuO nanoflakes. Mater. Sci. Eng. C-Mater. 2013, 33, 2020–2024. [Google Scholar] [CrossRef]
- Stohs, S.J.; Bagchi, D. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 1995, 18, 321–336. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.J.; Zhou, Y.; Ni, C.H.; Yu, L.M.; Li, C.C.; Yan, X.F.; Zhang, X.H. Research on electromagnetic wave absorption properties of zinc-based acrylate resins for marine antifouling coating. J. Alloys Compd. 2022, 900, 163285. [Google Scholar] [CrossRef]







| Bacterial Strains | E. coli | S. aureus | ||||||
|---|---|---|---|---|---|---|---|---|
| Samples | S0 | S2 | S4 | S5 | S0 | S2 | S4 | S5 |
| Numbers of living bacterial colonies | 580 | 143 | 11 | 23 | 380 | 66 | 4 | 14 |
| A/% | / | 75.3 | 98.1 | 96.0 | / | 82.6 | 98.9 | 96.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Hou, J. Preparation and Antibacterial Activity of Nano Copper Oxide- Loaded Zeolite 10X. Int. J. Mol. Sci. 2022, 23, 8421. https://doi.org/10.3390/ijms23158421
Ma Y, Hou J. Preparation and Antibacterial Activity of Nano Copper Oxide- Loaded Zeolite 10X. International Journal of Molecular Sciences. 2022; 23(15):8421. https://doi.org/10.3390/ijms23158421
Chicago/Turabian StyleMa, Yang, and Jin Hou. 2022. "Preparation and Antibacterial Activity of Nano Copper Oxide- Loaded Zeolite 10X" International Journal of Molecular Sciences 23, no. 15: 8421. https://doi.org/10.3390/ijms23158421