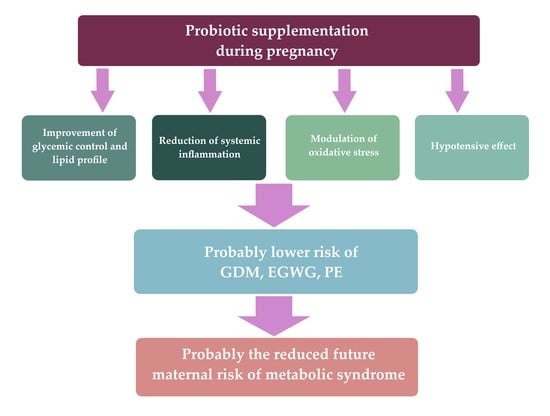
Effects of Probiotic Supplementation during Pregnancy on the Future Maternal Risk of Metabolic Syndrome
Abstract
:1. Introduction
2. Pregnancy as a Window for the Development of Metabolic Diseases
3. Therapeutic Applications of Probiotics
3.1. The Use of Probiotics in GDM and EGWG
3.2. The Use of Probiotics in PE
3.3. The Use of Probiotics in Obesity and Lipid Disorders
3.4. Probiotics and the Prevention of the Development of MS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reid, G.; Food and Agricultural Organization of the United Nation and the WHO. The Importance of Guidelines in the Development and Application of Probiotics. Curr. Pharm. Des. 2005, 11, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and Prebiotics in Intestinal Health and Disease: From Biology to the Clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Zhou, D.D.; Gan, R.Y.; Huang, S.Y.; Zhao, C.N.; Shang, A.; Xu, X.Y.; Li, H.B. Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review. Nutrients 2021, 13, 3211. [Google Scholar] [CrossRef] [PubMed]
- Zoumpopoulou, G.; Pot, B.; Tsakalidou, E.; Papadimitriou, K. Dairy Probiotics: Beyond the Role of Promoting Gut and Immune Health. Int. Dairy J. 2017, 67, 46–60. [Google Scholar] [CrossRef]
- Didari, T.; Solki, S.; Mozaffari, S.; Nikfar, S.; Abdollahi, M. A Systematic Review of the Safety of Probiotics. Expert Opin. Drug Saf. 2014, 13, 227–239. [Google Scholar] [CrossRef]
- Dugoua, J.J.; Machado, M.; Zhu, X.; Chen, X.; Koren, G.; Einarson, T.R. Probiotic Safety in Pregnancy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials of Lactobacillus, Bifidobacterium, and Saccharomyces spp. JOGC 2009, 31, 542–552. [Google Scholar] [CrossRef]
- Kijmanawat, A.; Panburana, P.; Reutrakul, S.; Tangshewinsirikul, C. Effects of Probiotic Supplements on Insulin Resistance in Gestational Diabetes Mellitus: A Double-Blind Randomized Controlled Trial. J. Diabetes Investig. 2019, 10, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Allen, S.J.; Jordan, S.; Storey, M.; Thornton, C.A.; Gravenor, M.; Garaiova, I.; Plummer, S.F.; Wang, D.; Morgan, G. Dietary Supplementation with Lactobacilli and Bifidobacteria Is Well Tolerated and Not Associated with Adverse Events during Late Pregnancy and Early Infancy. J. Nutr. 2010, 140, 483–488. [Google Scholar] [CrossRef]
- Elias, J.; Bozzo, P.; Einarson, A. Are Probiotics Safe for Use during Pregnancy and Lactation? Can. Fam. Physician 2011, 57, 299–301. [Google Scholar]
- Sotoudegan, F.; Daniali, M.; Hassani, S.; Nikfar, S.; Abdollahi, M. Reappraisal of Probiotics’ Safety in Human. Food Chem. Toxicol. 2019, 129, 22–29. [Google Scholar] [CrossRef]
- Homayoni, A.; Mehrabany, E.V.; Alipoor, B.; Mehrabany, L.V.; Javadi, M. Do probiotics act more efficiently in foods than in supplements? Nutrition 2012, 28, 733–736. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Shi, B. Gut Microbiota as a Potential Target of Metabolic Syndrome: The Role of Probiotics and Prebiotics. Cell Biosci. 2017, 7, 54. [Google Scholar] [CrossRef] [Green Version]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guarino, A.; Guandalini, S.; Lo Vecchio, A. Probiotics for Prevention and Treatment of Diarrhea. J. Clin. Gastroenterol. 2015, 49, 37. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Guevarra, R.B.; Kim, Y.T.; Kwon, J.; Kim, H.; Cho, J.H.; Kim, H.B.; Lee, J.H. Role of Probiotics in Human Gut Microbiome-Associated Diseases. J. Microbiol. Biotechnol. 2019, 29, 1335–1340. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-Bacterial Mutualism in the Human Intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. Human Gut Microbial Gene Catalogue Established by Metagenomic Sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. Metagenome-Wide Association Study of Gut Microbiota in Type 2 Diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Grenham, S.; Clarke, G.; Cryan, J.F.; Dinan, T.G. Brain-Gut-Microbe Communication in Health and Disease. Front. Physiol. 2011, 2, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Human Microbiome Project Consortium. Structure, Function and Diversity of the Healthy Human Microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Torres-Fuentes, C.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. The Microbiota-Gut-Brain Axis in Obesity. Lancet Gastroenterol. Hepatol. 2017, 2, 747–756. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. Brain-Gut-Microbiota Axis-Mood, Metabolism and Behaviour. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 69–70. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The Healthy Human Microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cani, P.D.; Delzenne, N.M. The Gut Microbiome as Therapeutic Target. Pharmacol. Ther. 2011, 130, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host Remodeling of the Gut Microbiome and Metabolic Changes during Pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef] [Green Version]
- Tilg, H.; Moschen, A.R. Food, Immunity, and the Microbiome. Gastroenterology 2015, 148, 1107–1119. [Google Scholar] [CrossRef]
- Gohir, W.; Whelan, F.J.; Surette, M.G.; Moore, C.; Schertzer, J.D.; Sloboda, D.M. Pregnancy-Related Changes in the Maternal Gut Microbiota Are Dependent upon the Mother’s Periconceptional Diet. Gut Microbes 2015, 6, 310–320. [Google Scholar] [CrossRef] [Green Version]
- Tune, J.D.; Goodwill, A.G.; Sassoon, D.J.; Mather, K.J. Cardiovascular Consequences of Metabolic Syndrome. Transl. Res. 2017, 183, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Tran, V.; De Silva, T.M.; Sobey, C.G.; Lim, K.; Drummond, G.R.; Vinh, A.; Jelinic, M. The Vascular Consequences of Metabolic Syndrome: Rodent Models, Endothelial Dysfunction, and Current Therapies. Front. Pharmacol. 2020, 11, 148. [Google Scholar] [CrossRef] [Green Version]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. Diagnosis and Management of the Metabolic Syndrome. Circulation 2005, 112, 285–290. [Google Scholar]
- Alberti, K.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the Metabolic Syndrome. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thanawala, U.; Divakar, H.; Jain, R.; Agarwal, M.M. Negotiating Gestational Diabetes Mellitus in India: A National Approach. Medicina 2021, 57, 942. [Google Scholar] [CrossRef] [PubMed]
- Alejandro, E.U.; Mamerto, T.P.; Chung, G.; Villavieja, A.; Gaus, N.L.; Morgan, E.; Pineda-Cortel, M.R.B. Gestational Diabetes Mellitus: A Harbinger of the Vicious Cycle of Diabetes. Int. J. Mol. Sci. 2020, 21, 5003. [Google Scholar] [CrossRef] [PubMed]
- Skórzyńska-Dziduszko, K.E.; Kimber-Trojnar, Ż.; Patro-Małysza, J.; Stenzel-Bembenek, A.; Oleszczuk, J.; Leszczyńska-Gorzelak, B. Heat Shock Proteins as a Potential Therapeutic Target in the Treatment of Gestational Diabetes Mellitus: What We Know so Far. Int. J. Mol. Sci. 2018, 19, 3205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Mendonça, E.L.S.S.; Fragoso, M.B.T.; de Oliveira, J.M.; Xavier, J.A.; Goulart, M.O.F.; de Oliveira, A.C.M. Gestational Diabetes Mellitus: The Crosslink among Inflammation, Nitroxidative Stress, Intestinal Microbiota and Alternative Therapies. Antioxidants 2022, 11, 129. [Google Scholar] [CrossRef]
- Kampmann, U.; Knorr, S.; Fuglsang, J.; Ovesen, P. Determinants of Maternal Insulin Resistance during Pregnancy: An Updated Overview. J. Diabetes Res. 2019, 2019, 5320156. [Google Scholar] [CrossRef] [Green Version]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef] [Green Version]
- Anonymous. ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet. Gynecol. 2018, 131, e49–e64. [Google Scholar] [CrossRef]
- Juan, J.; Yang, H. Prevalence, Prevention, and Lifestyle Intervention of Gestational Diabetes Mellitus in China. Int. J. Environ. Res. 2020, 17, 9517. [Google Scholar] [CrossRef]
- Nguyen, C.L.; Pham, N.M.; Binns, C.W.; Duong, D.V.; Lee, A.H. Prevalence of Gestational Diabetes Mellitus in Eastern and Southeastern Asia: A Systematic Review and Meta-Analysis. J. Diabetes Res. 2018, 2018, 6536974. [Google Scholar] [CrossRef] [Green Version]
- Dalfrà, M.G.; Burlina, S.; Del Vescovo, G.G.; Lapolla, A. Genetics and Epigenetics: New Insight on Gestational Diabetes Mellitus. Front. Endocrinol. 2020, 11, 602477. [Google Scholar] [CrossRef] [PubMed]
- Tranidou, A.; Dagklis, T.; Tsakiridis, I.; Siargkas, A.; Apostolopoulou, A.; Mamopoulos, A.; Goulis, D.G.; Chourdakis, M. Risk of Developing Metabolic Syndrome after Gestational Diabetes Mellitus—A Systematic Review and Meta-Analysis. J. Endocrinol. Investig. 2021, 44, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Ruszała, M.; Niebrzydowska, M.; Pilszyk, A.; Kimber-Trojnar, Ż.; Trojnar, M.; Leszczyńska-Gorzelak, B. Novel Biomolecules in the Pathogenesis of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2021, 22, 11578. [Google Scholar] [CrossRef]
- Trojnar, M.; Patro-Małysza, J.; Kimber-Trojnar, Ż.; Leszczyńska-Gorzelak, B.; Mosiewicz, J. Associations between Fatty Acid-Binding Protein 4–A Proinflammatory Adipokine and Insulin Resistance, Gestational and Type 2 Diabetes Mellitus. Cells 2019, 8, 227. [Google Scholar] [CrossRef] [Green Version]
- Patro-Małysza, J.; Trojnar, M.; Skórzyńska-Dziduszko, K.E.; Kimber-Trojnar, Ż.; Darmochwał-Kolarz, D.; Czuba, M.; Leszczyńska-Gorzelak, B. Leptin and Ghrelin in Excessive Gestational Weight Gain-Association between Mothers and Offspring. Int. J. Mol. Sci. 2019, 20, 2398. [Google Scholar] [CrossRef] [Green Version]
- Kimber-Trojnar, Ż.; Patro-Małysza, J.; Trojnar, M.; Skórzyńska-Dziduszko, K.E.; Bartosiewicz, J.; Oleszczuk, J.; Leszczyńska-Gorzelak, B. Fatty Acid-Binding Protein 4—An “Inauspicious” Adipokine—In Serum and Urine of Post-Partum Women with Excessive Gestational Weight Gain and Gestational Diabetes Mellitus. J. Clin. Med. 2018, 7, 505. [Google Scholar] [CrossRef] [Green Version]
- Florian, A.R.; Cruciat, G.; Pop, R.M.; Staicu, A.; Daniel, M.; Florin, S. Predictive Role of Altered Leptin, Adiponectin and 3-Carboxy-4-Methyl-5-Propyl-2-Furanpropanoic Acid Secretion in Gestational Diabetes Mellitus. Exp. Ther. Med. 2021, 21, 520. [Google Scholar] [CrossRef]
- Kapustin, R.V.; Chepanov, S.V.; Babakov, V.N.; Rogovskaya, N.Y.; Kopteeva, E.V.; Alekseenkova, E.N.; Arzhanova, O.N. Maternal serum leptin, adiponectin, resistin and monocyte chemoattractant protein-1 levels in different types of diabetes mellitus. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 254, 284–291. [Google Scholar] [CrossRef]
- Aslfalah, H.; Jamilian, M.; Khosrowbeygi, A. Elevation of the Adiponectin/Leptin Ratio in Women with Gestational Diabetes Mellitus after Supplementation with Alpha-Lipoic Acid. Gynecol. Endocrinol. 2019, 35, 271–275. [Google Scholar] [CrossRef]
- Meriç, P.; Özçaka, Ö.; Ceyhan-Öztürk, B.; Akcal, A.; Nalbantsoy, A.; Buduneli, N. Salivary Adiponectin and Leptin Levels Are Increased in Women with Gestational Diabetes Mellitus and Gingival Inflammation. Oral Health Prev. Dent. 2018, 16, 541–547. [Google Scholar]
- Ruszała, M.; Pilszyk, A.; Niebrzydowska, M.; Kimber-Trojnar, Ż.; Trojnar, M.; Leszczyńska-Gorzelak, B. Novel Biomolecules in the Pathogenesis of Gestational Diabetes Mellitus 2.0. Int. J. Mol. Sci. 2022, 23, 4364. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, A.; Vilariño-García, T.; Guadix, P.; Dueñas, J.L.; Sánchez-Margalet, V. Leptin and Nutrition in Gestational Diabetes. Nutrients 2020, 12, 1970. [Google Scholar] [CrossRef] [PubMed]
- Pheiffer, C.; Dias, S.; Jack, B.; Malaza, N.; Adam, S. Adiponectin as a Potential Biomarker for Pregnancy Disorders. Int. J. Mol. Sci. 2021, 22, 1326. [Google Scholar] [CrossRef] [PubMed]
- Patro-Małysza, J.; Trojnar, M.; Kimber-Trojnar, Ż.; Mierzyński, R.; Bartosiewicz, J.; Oleszczuk, J.; Leszczyńska-Gorzelak, B. FABP4 in Gestational Diabetes—Association between Mothers and Offspring. J. Clin. Med. 2019, 8, 285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzo-Almorós, A.; Hang, T.; Peiró, C.; Soriano-Guillén, L.; Egido, J.; Tuñón, J.; Lorenzo, Ó. Predictive and Diagnostic Biomarkers for Gestational Diabetes and Its Associated Metabolic and Cardiovascular Diseases. Cardiovasc. Diabetol. 2019, 18, 140. [Google Scholar] [CrossRef]
- Nguyen-Ngo, C.; Jayabalan, N.; Salomon, C.; Lappas, M. Molecular Pathways Disrupted by Gestational Diabetes Mellitus. J. Mol. Endocrinol. 2019, 63, 51–72. [Google Scholar] [CrossRef] [Green Version]
- Szymczak-Pajor, I.; Śliwińska, A. Analysis of Association between Vitamin D Deficiency and Insulin Resistance. Nutrients 2019, 11, 794. [Google Scholar] [CrossRef] [Green Version]
- Wimalawansa, S.J. Associations of Vitamin D with Insulin Resistance, Obesity, Type 2 Diabetes, and Metabolic Syndrome. J. Steroid. Biochem. Mol. Biol. 2018, 175, 177–189. [Google Scholar] [CrossRef]
- Said, J.; Lagat, D.; Kimaina, A.; Oduor, C. Beta Cell Function, Insulin Resistance and Vitamin D Status among Type 2 Diabetes Patients in Western Kenya. Sci. Rep. 2021, 11, 4084. [Google Scholar] [CrossRef]
- Ebadi, S.A.; Sharifi, L.; Rashidi, E.; Ebadi, S.S.; Khalili, S.; Sadeghi, S.; Afzali, N.; Shiri, S.M. Supplementation with Vitamin D and Insulin Homeostasis in Healthy Overweight and Obese Adults: A Randomized Clinical Trial. Obes. Res. Clin. Pract. 2021, 15, 256–261. [Google Scholar] [CrossRef]
- Skórzyńska-Dziduszko, K.E.; Kimber-Trojnar, Ż.; Patro-Małysza, J.; Olszewska, A.; Zaborowski, T.; Małecka-Massalska, T. An Interplay between Obesity and Inflammation in Gestational Diabetes Mellitus. Curr. Pharm. Biotechnol. 2016, 17, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Ornoy, A.; Becker, M.; Weinstein-Fudim, L.; Ergaz, Z. Diabetes during Pregnancy: A Maternal Disease Complicating the Course of Pregnancy with Long-Term Deleterious Effects on the Offspring. A Clinical Review. Int. J. Mol. Sci. 2021, 22, 2965. [Google Scholar] [CrossRef] [PubMed]
- Patro-Malysza, J.; Kimber-Trojnar, Z.; Skorzynska-Dziduszko, K.; Marciniak, B.; Darmochwal-Kolarz, D.; Bartosiewicz, J.; Leszczynska-Gorzelak, B.; Oleszczuk, J. The Impact of Substance P on the Pathogenesis of Insulin Resistance Leading to Gestational Diabetes. Curr. Pharm. Biotechnol. 2014, 15, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Trojnar, M.; Patro-Małysza, J.; Kimber-Trojnar, Ż.; Czuba, M.; Mosiewicz, J.; Leszczyńska-Gorzelak, B. Vaspin in Serum and Urine of Post-Partum Women with Excessive Gestational Weight Gain. Medicina 2019, 55, 76. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xiao, C.M.; Zhang, Y.; Chen, Q.; Zhang, X.Q.; Li, X.F.; Shao, R.Y.; Gao, Y.M. Factors Associated with Gestational Diabetes Mellitus: A Meta-Analysis. J. Diabetes Res. 2021, 2021, 6692695. [Google Scholar] [CrossRef]
- Davidson, K.W.; Barry, M.J.; Mangione, C.M.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Kubik, M.; Li, L.; et al. Screening for Gestational Diabetes: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 326, 531–538. [Google Scholar]
- Johns, E.C.; Denison, F.C.; Norman, J.E.; Reynolds, R.M. Gestational Diabetes Mellitus: Mechanisms, Treatment, and Complications. Trends Endocrinol. Metab. 2018, 29, 743–754. [Google Scholar] [CrossRef]
- Franzago, M.; Fraticelli, F.; Stuppia, L.; Vitacolonna, E. Nutrigenetics, Epigenetics and Gestational Diabetes: Consequences in Mother and Child. Epigenetics 2019, 14, 215–235. [Google Scholar] [CrossRef] [Green Version]
- Farahvar, S.; Walfisch, A.; Sheiner, E. Gestational Diabetes Risk Factors and Long-Term Consequences for Both Mother and Offspring: A Literature Review. Expert Rev. Endocrinol. Metab. 2019, 14, 63–74. [Google Scholar] [CrossRef]
- Wacker-Gussmann, A.; Schopen, J.; Engelhard, J.; Sitzberger, C.; Lienert, N.; Ewert, P.; Müller, A.; Schmidt, G.; Oberhoffer-Fritz, R.; Lobmaier, S.M. The Impact of Gestational Diabetes in Pregnancy on the Cardiovascular System of Children at One Year of Age. J. Clin. Med. 2021, 10, 5839. [Google Scholar] [CrossRef]
- Wu, P.; Gulati, M.; Kwok, C.S.; Wong, C.W.; Narain, A.; O’Brien, S.; Chew-Graham, C.A.; Verma, G.; Kadam, U.T.; Mamas, M.A. Preterm Delivery and Future Risk of Maternal Cardiovascular Disease: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2018, 7, 007809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nijs, H.; Benhalima, K. Gestational Diabetes Mellitus and the Long-Term Risk for Glucose Intolerance and Overweight in the Offspring: A Narrative Review. J. Clin. Med. 2020, 9, 599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, G.N.; Louis, J.M.; Saade, G.R. Pregnancy and the Postpartum Period as an Opportunity for Cardiovascular Risk Identification and Management. Obstet. Gynecol. 2019, 134, 851–862. [Google Scholar] [CrossRef]
- Bentley-Lewis, R. Gestational Diabetes Mellitus: An Opportunity of a Lifetime. Lancet 2009, 373, 1738–1740. [Google Scholar] [CrossRef] [Green Version]
- Hanna, F.W.; Duff, C.J.; Shelley-Hitchen, A.; Hodgson, E.; Fryer, A.A. Diagnosing Gestational Diabetes Mellitus: Implications of Recent Changes in Diagnostic Criteria and Role of Glycated Haemoglobin (HbA1c). Clin. Med. 2017, 17, 108–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herath, H.; Herath, R.; Wickremasinghe, R. Gestational Diabetes Mellitus and Risk of Type 2 Diabetes 10 Years after the Index Pregnancy in Sri Lankan Women—A Community Based Retrospective Cohort Study. PLoS ONE 2017, 12, e0179647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasain, Z.; Mokhtar, N.M.; Kamaruddin, N.A.; Mohamed Ismail, N.A.; Razalli, N.H.; Gnanou, J.V.; Raja Ali, R.A. Gut Microbiota and Gestational Diabetes Mellitus: A Review of Host-Gut Microbiota Interactions and Their Therapeutic Potential. Front. Cell. Infect. Microbiol. 2020, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, S.F.; Hedderson, M.M.; Feng, J.; Davenport, E.R.; Gunderson, E.P.; Ferrara, A. Change in Body Mass Index between Pregnancies and the Risk of Gestational Diabetes in a Second Pregnancy. Obstet. Gynecol. 2011, 117, 1323–1330. [Google Scholar] [CrossRef] [Green Version]
- Bellamy, L.; Casas, J.P.; Hingorani, A.D.; Williams, D. Type 2 Diabetes Mellitus after Gestational Diabetes: A Systematic Review and Meta-Analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef]
- Can, B.; Çiftçi, S.; Yenidünya, G.; Dinççağ, N. Risk factors predicting the development of diabetes mellitus and metabolic syndrome following gestational diabetes mellitus. Turk. J. Med. Sci. 2021, 51, 595–603. [Google Scholar] [CrossRef]
- Barker, J.; Su, F.; Alwan, N.A. Risk Factors for Type 2 Diabetes after Gestational Diabetes: A Population-Based Cohort Study. Lancet 2017, 390, 21. [Google Scholar] [CrossRef]
- Gunderson, E.P.; Chiang, V.; Pletcher, M.J.; Jacobs, D.R.; Quesenberry, C.P.; Sidney, S.; Lewis, C.E. History of Gestational Diabetes Mellitus and Future Risk of Atherosclerosis in Mid-life: The Coronary Artery Risk Development in Young Adults Study. J. Am. Heart Assoc. 2014, 3, 000490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Retnakaran, R.; Shah, B.R. Role of Type 2 Diabetes in Determining Retinal, Renal, and Cardiovascular Outcomes in Women With Previous Gestational Diabetes Mellitus. Diabetes Care 2017, 40, 101–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puhkala, J.; Raitanen, J.; Kolu, P.; Tuominen, P.; Husu, P.; Luoto, R. Metabolic Syndrome in Finnish Women 7 Years after a Gestational Diabetes Prevention Trial. BMJ Open 2017, 7, 014565. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, K.; Nielsen, M.F.; Kallfa, E.; Dubietyte, G.; Lauszus, F.F. Metabolic Syndrome in Women with Previous Gestational Diabetes. Sci. Rep. 2021, 11, 11558. [Google Scholar] [CrossRef]
- Pathirana, M.M.; Lassi, Z.S.; Ali, A.; Arstall, M.A.; Roberts, C.T.; Andraweera, P.H. Association between Metabolic Syndrome and Gestational Diabetes Mellitus in Women and Their Children: A Systematic Review and Meta-Analysis. Endocrine 2021, 71, 310–320. [Google Scholar] [CrossRef]
- Hakkarainen, H.; Huopio, H.; Cederberg, H.; Voutilainen, R.; Heinonen, S. Future Risk of Metabolic Syndrome in Women with a Previous LGA Delivery Stratified by Gestational Glucose Tolerance: A Prospective Cohort Study. BMC Pregnancy Childbirth 2018, 18, 326. [Google Scholar] [CrossRef] [Green Version]
- Bo, S.; Menato, G.; Gallo, M.L.; Bardelli, C.; Lezo, A.; Signorile, A.; Gambino, R.; Cassader, M.; Massobrio, M.; Pagano, G. Mild Gestational Hyperglycemia, the Metabolic Syndrome and Adverse Neonatal Outcomes. Acta Obstet. Gynecol. Scand. 2004, 83, 335–340. [Google Scholar] [CrossRef]
- Barquiel, B.; Herranz, L.; Hillman, N.; Burgos, M.Á.; Pallardo, L.F. Prepregnancy Body Mass Index and Prenatal Fasting Glucose Are Effective Predictors of Early Postpartum Metabolic Syndrome in Spanish Mothers with Gestational Diabetes. Metab. Syndr. Relat. Disord. 2014, 12, 457–463. [Google Scholar] [CrossRef]
- Green, M.; Arora, K.; Prakash, S. Microbial Medicine: Prebiotic and Probiotic Functional Foods to Target Obesity and Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 2890. [Google Scholar] [CrossRef]
- Ferrer, M.; Ruiz, A.; Lanza, F.; Haange, S.B.; Oberbach, A.; Till, H.; Bargiela, R.; Campoy, C.; Segura, M.T.; Richter, M.; et al. Microbiota from the Distal Guts of Lean and Obese Adolescents Exhibit Partial Functional Redundancy besides Clear Differences in Community Structure. Environ. Microbiol. 2013, 15, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Sanz, Y.; Rastmanesh, R.; Agostoni, C.; Agostonic, C. Understanding the Role of Gut Microbes and Probiotics in Obesity: How Far Are We? Pharmacol. Res. 2013, 69, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Raoult, D. Probiotics and Obesity: A Link? Nat. Rev. Microbiol. 2009, 7, 616. [Google Scholar] [CrossRef] [PubMed]
- Fabersani, E.; Abeijon-Mukdsi, M.C.; Ross, R.; Medina, R.; González, S.; Gauffin-Cano, P. Specific Strains of Lactic Acid Bacteria Differentially Modulate the Profile of Adipokines In Vitro. Front. Immunol. 2017, 8, 266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frigolet, M.E.; Gutiérrez-Aguilar, R. The Colors of Adipose Tissue. Gac. Med. Mex. 2020, 156, 142–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose Tissue Inflammation and Metabolic Dysfunction in Obesity. Am. J. Physiol. Cell Physiol. 2021, 320, 375–391. [Google Scholar] [CrossRef]
- Röszer, T. Adipose Tissue Immunometabolism and Apoptotic Cell Clearance. Cells 2021, 10, 2288. [Google Scholar] [CrossRef]
- Grant, R.W.; Dixit, V.D. Adipose Tissue as an Immunological Organ. Obesity 2015, 23, 512–518. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Shen, S.; Sun, L.; Yang, H.; Jin, B.; Cao, X. Metabolic Syndrome Risk after Gestational Diabetes: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e87863. [Google Scholar] [CrossRef] [Green Version]
- Huopio, H.; Cederberg, H.; Vangipurapu, J.; Hakkarainen, H.; Pääkkönen, M.; Kuulasmaa, T.; Heinonen, S.; Laakso, M. Association of Risk Variants for Type 2 Diabetes and Hyperglycemia with Gestational Diabetes. Eur. J. Endocrinol. 2013, 169, 291–297. [Google Scholar] [CrossRef] [Green Version]
- Oskovi-Kaplan, Z.A.; Ozgu-Erdinc, A.S. Management of Gestational Diabetes Mellitus. Adv. Exp. Med. Biol. 2021, 1307, 257–272. [Google Scholar] [PubMed]
- Homayouni, A.; Bagheri, N.; Mohammad-Alizadeh-Charandabi, S.; Kashani, N.; Mobaraki-Asl, N.; Mirghafurvand, M.; Asgharian, H.; Ansari, F.; Pourjafar, H. Prevention of Gestational Diabetes Mellitus (GDM) and Probiotics: Mechanism of Action: A Review. Curr. Diabetes Rev. 2020, 16, 538–545. [Google Scholar] [CrossRef]
- Ferrocino, I.; Ponzo, V.; Gambino, R.; Zarovska, A.; Leone, F.; Monzeglio, C.; Goitre, I.; Rosato, R.; Romano, A.; Grassi, G.; et al. Changes in the Gut Microbiota Composition during Pregnancy in Patients with Gestational Diabetes Mellitus (GDM). Sci. Rep. 2018, 8, 12216. [Google Scholar] [CrossRef] [PubMed]
- Crusell, M.K.W.; Hansen, T.H.; Nielsen, T.; Allin, K.H.; Rühlemann, M.C.; Damm, P.; Vestergaard, H.; Rørbye, C.; Jørgensen, N.R.; Christiansen, O.B.; et al. Gestational Diabetes Is Associated with Change in the Gut Microbiota Composition in Third Trimester of Pregnancy and Postpartum. Microbiome 2018, 6, 89. [Google Scholar] [CrossRef]
- Kuang, Y.S.; Lu, J.H.; Li, S.H.; Li, J.H.; Yuan, M.Y.; He, J.R.; Chen, N.N.; Xiao, W.Q.; Shen, S.Y.; Qiu, L.; et al. Connections between the Human Gut Microbiome and Gestational Diabetes Mellitus. Gigascience 2017, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Pan, L.L.; Lv, S.; Yang, Q.; Zhang, H.; Chen, W.; Lv, Z.; Sun, J. Alterations of Gut Microbiota and Blood Lipidome in Gestational Diabetes Mellitus with Hyperlipidemia. Front. Physiol. 2019, 10, 1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Zhang, M.; Zhang, J.; Sun, Z.; Ran, L.; Ban, Y.; Wang, B.; Hou, X.; Zhai, S.; Ren, L.; et al. Differential Intestinal and Oral Microbiota Features Associated with Gestational Diabetes and Maternal Inflammation. Am. J. Physiol. Endocrinol. Metab. 2020, 319, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Cortez, R.V.; Taddei, C.R.; Sparvoli, L.G.; Ângelo, A.G.S.; Padilha, M.; Mattar, R.; Daher, S. Microbiome and Its Relation to Gestational Diabetes. Endocrine 2019, 64, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Mokkala, K.; Paulin, N.; Houttu, N.; Koivuniemi, E.; Pellonperä, O.; Khan, S.; Pietilä, S.; Tertti, K.; Elo, L.L.; Laitinen, K. Metagenomics Analysis of Gut Microbiota in Response to Diet Intervention and Gestational Diabetes in Overweight and Obese Women: A Randomised, Double-Blind, Placebo-Controlled Clinical Trial. Gut 2021, 70, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Luoto, R.; Laitinen, K.; Nermes, M.; Isolauri, E. Impact of Maternal Probiotic-Supplemented Dietary Counselling on Pregnancy Outcome and Prenatal and Postnatal Growth: A Double-Blind, Placebo-Controlled Study. Br. J. Nutr. 2010, 103, 1792–1799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nabhani, Z.; Hezaveh, S.J.G.; Razmpoosh, E.; Asghari-Jafarabadi, M.; Gargari, B.P. The Effects of Synbiotic Supplementation on Insulin Resistance/Sensitivity, Lipid Profile and Total Antioxidant Capacity in Women with Gestational Diabetes Mellitus: A Randomized Double Blind Placebo Controlled Clinical Trial. Diabetes Res. Clin. Pract. 2018, 138, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Sahhaf Ebrahimi, F.; Homayouni Rad, A.; Mosen, M.; Abbasalizadeh, F.; Tabrizi, A.; Khalili, L. Effect of L. Acidophilus and B. Lactis on Blood Glucose in Women with Gestational Diabetes Mellitus: A Randomized Placebo-Controlled Trial. Diabetol. Metab. Syndr. 2019, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, K.; Poussa, T.; Isolauri, E. Probiotics and Dietary Counselling Contribute to Glucose Regulation during and after Pregnancy: A Randomised Controlled Trial. Br. J. Nutr. 2008, 101, 1679–1687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babadi, M.; Khorshidi, A.; Aghadavood, E.; Samimi, M.; Kavossian, E.; Bahmani, F.; Mafi, A.; Shafabakhsh, R.; Satari, M.; Asemi, Z. The Effects of Probiotic Supplementation on Genetic and Metabolic Profiles in Patients with Gestational Diabetes Mellitus: A Randomized, Double-Blind, Placebo-Controlled Trial. Probiotics Antimicrob. Proteins 2019, 11, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Okesene-Gafa, K.A.; Moore, A.E.; Jordan, V.; McCowan, L.; Crowther, C.A. Probiotic Treatment for Women with Gestational Diabetes to Improve Maternal and Infant Health and Well-being. Cochrane Database Syst. Rev. 2020, 2020, 012970. [Google Scholar]
- Davidson, S.J.; Barrett, H.L.; Price, S.A.; Callaway, L.K.; Dekker Nitert, M. Probiotics for Preventing Gestational Diabetes. Cochrane Database Syst. Rev. 2021, 4, 9951. [Google Scholar]
- Taylor, B.L.; Woodfall, G.E.; Sheedy, K.E.; O’Riley, M.L.; Rainbow, K.A.; Bramwell, E.L.; Kellow, N.J. Effect of Probiotics on Metabolic Outcomes in Pregnant Women with Gestational Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2017, 9, 461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masulli, M.; Vitacolonna, E.; Fraticelli, F.; Della Pepa, G.; Mannucci, E.; Monami, M. Effects of Probiotic Supplementation during Pregnancy on Metabolic Outcomes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diabetes Res. Clin. Pract. 2020, 162, 108111. [Google Scholar] [CrossRef]
- Pellonperä, O.; Mokkala, K.; Houttu, N.; Vahlberg, T.; Koivuniemi, E.; Tertti, K.; Rönnemaa, T.; Laitinen, K. Efficacy of Fish Oil and/or Probiotic Intervention on the Incidence of Gestational Diabetes Mellitus in an At-Risk Group of Overweight and Obese Women: A Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Diabetes Care 2019, 42, 1009–1017. [Google Scholar] [CrossRef]
- Callaway, L.K.; McIntyre, H.D.; Barrett, H.L.; Foxcroft, K.; Tremellen, A.; Lingwood, B.E.; Tobin, J.M.; Wilkinson, S.; Kothari, A.; Morrison, M.; et al. Probiotics for the Prevention of Gestational Diabetes Mellitus in Overweight and Obese Women: Findings From the SPRING Double-Blind Randomized Controlled Trial. Diabetes Care 2019, 42, 364–371. [Google Scholar] [CrossRef] [Green Version]
- Lindsay, K.L.; Brennan, L.; Kennelly, M.A.; Maguire, O.C.; Smith, T.; Curran, S.; Coffey, M.; Foley, M.E.; Hatunic, M.; Shanahan, F.; et al. Impact of Probiotics in Women with Gestational Diabetes Mellitus on Metabolic Health: A Randomized Controlled Trial. Am. J. Obstet. Gynecol. 2015, 212, 496. [Google Scholar] [PubMed]
- Karamali, M.; Dadkhah, F.; Sadrkhanlou, M.; Jamilian, M.; Ahmadi, S.; Tajabadi-Ebrahimi, M.; Jafari, P.; Asemi, Z. Effects of Probiotic Supplementation on Glycaemic Control and Lipid Profiles in Gestational Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Diabetes Metab. 2016, 42, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Jafarnejad, S.; Saremi, S.; Jafarnejad, F.; Arab, A. Effects of a Multispecies Probiotic Mixture on Glycemic Control and Inflammatory Status in Women with Gestational Diabetes: A Randomized Controlled Clinical Trial. J. Nutr. Metab. 2016, 2016, 5190846. [Google Scholar] [CrossRef] [Green Version]
- Dolatkhah, N.; Hajifaraji, M.; Abbasalizadeh, F.; Aghamohammadzadeh, N.; Mehrabi, Y.; Abbasi, M.M. Is There a Value for Probiotic Supplements in Gestational Diabetes Mellitus? A Randomized Clinical Trial. J. Health Popul. Nutr. 2015, 33, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paszti-Gere, E.; Szeker, K.; Csibrik-Nemeth, E.; Csizinszky, R.; Marosi, A.; Palocz, O.; Farkas, O.; Galfi, P. Metabolites of Lactobacillus Plantarum 2142 Prevent Oxidative Stress-Induced Overexpression of Proinflammatory Cytokines in IPEC-J2 Cell Line. Inflammation 2012, 35, 1487–1499. [Google Scholar] [CrossRef]
- Yadav, H.; Lee, J.H.; Lloyd, J.; Walter, P.; Rane, S.G. Beneficial Metabolic Effects of a Probiotic via Butyrate-Induced GLP-1 Hormone Secretion. J. Biol. Chem. 2013, 288, 25088–25097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ejtahed, H.S.; Mohtadi-Nia, J.; Homayouni-Rad, A.; Niafar, M.; Asghari-Jafarabadi, M.; Mofid, V. Probiotic Yogurt Improves Antioxidant Status in Type 2 Diabetic Patients. Nutrition 2012, 28, 539–543. [Google Scholar] [CrossRef]
- Kim, Y.A.; Keogh, J.B.; Clifton, P.M. Probiotics, Prebiotics, Synbiotics and Insulin Sensitivity. Nutr. Res. Rev. 2018, 31, 35–51. [Google Scholar] [CrossRef]
- Gomes, A.C.; Bueno, A.A.; de Souza, R.G.M.; Mota, J.F. Gut Microbiota, Probiotics and Diabetes. Nutr. J. 2014, 13, 60. [Google Scholar] [CrossRef] [Green Version]
- Sichetti, M.; De Marco, S.; Pagiotti, R.; Traina, G.; Pietrella, D. Anti-Inflammatory Effect of Multistrain Probiotic Formulation (L. rhamnosus, B. lactis, and B. longum). Nutrition 2018, 53, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, X.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus Casei CCFM419 Attenuates Type 2 Diabetes via a Gut Microbiota Dependent Mechanism. Food Funct. 2017, 8, 3155–3164. [Google Scholar] [CrossRef]
- Tamtaji, O.R.; Kouchaki, E.; Salami, M.; Aghadavod, E.; Akbari, E.; Tajabadi-Ebrahimi, M.; Asemi, Z. The Effects of Probiotic Supplementation on Gene Expression Related to Inflammation, Insulin, and Lipids in Patients With Multiple Sclerosis: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Coll. Nutr. 2017, 36, 660–665. [Google Scholar] [CrossRef]
- Krumbeck, J.A.; Rasmussen, H.E.; Hutkins, R.W.; Clarke, J.; Shawron, K.; Keshavarzian, A.; Walter, J. Probiotic Bifidobacterium Strains and Galactooligosaccharides Improve Intestinal Barrier Function in Obese Adults but Show No Synergism When Used Together as Synbiotics. Microbiome 2018, 6, 121. [Google Scholar] [CrossRef]
- Badehnoosh, B.; Karamali, M.; Zarrati, M.; Jamilian, M.; Bahmani, F.; Tajabadi-Ebrahimi, M.; Jafari, P.; Rahmani, E.; Asemi, Z. The Effects of Probiotic Supplementation on Biomarkers of Inflammation, Oxidative Stress and Pregnancy Outcomes in Gestational Diabetes. J. Matern. Fetal Neonatal Med. 2018, 31, 1128–1136. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zheng, J.; Shi, W.; Du, N.; Xu, X.; Zhang, Y.; Ji, P.; Zhang, F.; Jia, Z.; Wang, Y.; et al. Dysbiosis of Maternal and Neonatal Microbiota Associated with Gestational Diabetes Mellitus. Gut 2018, 67, 1614–1625. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, S.; Feng, Y.; Song, Y.; Lv, N.; Liu, F.; Zhang, X.; Wang, S.; Wei, Y.; Li, S.; et al. Perturbations of Gut Microbiota in Gestational Diabetes Mellitus Patients Induce Hyperglycemia in Germ-Free Mice. J. Dev. Orig. Health Dis. 2020, 11, 580–588. [Google Scholar] [CrossRef]
- Ye, G.; Zhang, L.; Wang, M.; Chen, Y.; Gu, S.; Wang, K.; Leng, J.; Gu, Y.; Xie, X. The Gut Microbiota in Women Suffering from Gestational Diabetes Mellitus with the Failure of Glycemic Control by Lifestyle Modification. J. Diabetes Res. 2019, 2019, 6081248. [Google Scholar] [CrossRef]
- Zheng, W.; Xu, Q.; Huang, W.; Yan, Q.; Chen, Y.; Zhang, L.; Tian, Z.; Liu, T.; Yuan, X.; Liu, C.; et al. Gestational Diabetes Mellitus Is Associated with Reduced Dynamics of Gut Microbiota during the First Half of Pregnancy. mSystems 2020, 5, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Wu, W.; Liu, Z.; Cong, Y. Microbiota Metabolite Short Chain Fatty Acids, GCPR, and Inflammatory Bowel Diseases. J. Gastroenterol. 2017, 52, 1–8. [Google Scholar] [CrossRef]
- Hu, J.; Lin, S.; Zheng, B.; Cheung, P.C.K. Short-Chain Fatty Acids in Control of Energy Metabolism. Crit. Rev. Food Sci. Nutr. 2018, 58, 1243–1249. [Google Scholar] [CrossRef]
- Nagpal, R.; Wang, S.; Ahmadi, S.; Hayes, J.; Gagliano, J.; Subashchandrabose, S.; Kitzman, D.W.; Becton, T.; Read, R.; Yadav, H. Human-Origin Probiotic Cocktail Increases Short-Chain Fatty Acid Production via Modulation of Mice and Human Gut Microbiome. Sci. Rep. 2018, 8, 12649. [Google Scholar] [CrossRef] [Green Version]
- Czajkowska, A.; Szponar, B. Short Chain Fatty Acids (SCFA), the Products of Gut Bacteria Metabolism and Their Role in the Host. Postepy Hig. Med. Dosw. 2018, 72, 131–142. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, Y.; Chen, H.; Wei, H.; Wan, C. Potential of Lactobacillus Plantarum ZDY2013 and Bifidobacterium Bifidum WBIN03 in Relieving Colitis by Gut Microbiota, Immune, and Anti-Oxidative Stress. Can. J. Microbiol. 2018, 64, 327–337. [Google Scholar] [CrossRef]
- Huang, Y.C.; Wu, B.H.; Chu, Y.L.; Chang, W.C.; Wu, M.C. Effects of Tempeh Fermentation with Lactobacillus Plantarum and Rhizopus Oligosporus on Streptozotocin-Induced Type II Diabetes Mellitus in Rats. Nutrients 2018, 10, 1143. [Google Scholar] [CrossRef] [Green Version]
- Mu, W.C.; VanHoosier, E.; Elks, C.M.; Grant, R.W. Long-Term Effects of Dietary Protein and Branched-Chain Amino Acids on Metabolism and Inflammation in Mice. Nutrients 2018, 10, 918. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Thonusin, C.; Chattipakorn, N.; Chattipakorn, S.C. Impacts of Gut Microbiota on Gestational Diabetes Mellitus: A Comprehensive Review. Eur. J. Nutr. 2021, 60, 2343–2360. [Google Scholar] [CrossRef]
- Całyniuk, B.; Grochowska-Niedworok, E.; Walkiewicz, K.W.; Kawecka, S.; Popiołek, E.; Fatyga, E. Dialdehyd Malonowy-Produkt Peroksydacji Lipidów Jako Marker Zaburzeń Homeostazy i Wieku. Ann. Acad. Med. Silesiensis 2016, 70, 224–228. [Google Scholar] [CrossRef]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative Stress and Reactive Oxygen Species in Endothelial Dysfunction Associated with Cardiovascular and Metabolic Diseases. Vascul. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Okesene-Gafa, K.A.M.; Li, M.; McKinlay, C.J.D.; Taylor, R.S.; Rush, E.C.; Wall, C.R.; Wilson, J.; Murphy, R.; Taylor, R.; Thompson, J.M.D.; et al. Effect of Antenatal Dietary Interventions in Maternal Obesity on Pregnancy Weight-Gain and Birthweight: Healthy Mums and Babies (HUMBA) Randomized Trial. AJOG 2019, 221, 152. [Google Scholar] [CrossRef]
- Aung, W.; Saw, L.; Sweet, L. An Integrative Review of Interventions for Limiting Gestational Weight Gain in Pregnant Women Who Are Overweight or Obese. Women Birth 2022, 35, 108–126. [Google Scholar] [CrossRef]
- Rafeeinia, A.; Tabandeh, A.; Khajeniazi, S.; Marjani, A. Metabolic Syndrome in Preeclampsia Women in Gorgan. Open Biochem. J. 2014, 8, 94–99. [Google Scholar]
- Steegers, E.A.; Dadelszen, P.; Duvekot, J.J.; Pijnenborg, R. Pre-Eclampsia. Lancet 2010, 376, 631–644. [Google Scholar] [CrossRef]
- Dildy, G.A.; Belfort, M.A.; Smulian, J.C. Preeclampsia Recurrence and Prevention. Semin. Perinatol. 2007, 31, 135–141. [Google Scholar] [CrossRef]
- Roberts, J.M.; Pearson, G.; Cutler, J.; Lindheimer, M. Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension 2003, 41, 437–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duley, L. The Global Impact of Pre-Eclampsia and Eclampsia. Semin. Perinatol. 2009, 33, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, L.; Casas, J.P.; Hingorani, A.D.; Williams, D.J. Pre-Eclampsia and Risk of Cardiovascular Disease and Cancer in Later Life: Systematic Review and Meta-Analysis. BMJ 2007, 335, 974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romundstad, P.R.; Magnussen, E.B.; Smith, G.D.; Vatten, L.J. Hypertension in Pregnancy and Later Cardiovascular Risk: Common Antecedents? Circulation 2010, 122, 579–584. [Google Scholar] [CrossRef] [Green Version]
- Heidema, W.M.; Scholten, R.R.; van Drongelen, J.; Spaanderman, M.E.A. Metabolic Syndrome after Preeclamptic Pregnancy: A Longitudinal Cohort Study. J. Womens Health 2019, 28, 357–362. [Google Scholar] [CrossRef]
- Rangaswami, J.; Naranjo, M.; McCullough, P.A. Preeclampsia as a Form of Type 5 Cardiorenal Syndrome: An Underrecognized Entity in Women’s Cardiovascular Health. Cardiorenal Med. 2018, 8, 160–172. [Google Scholar] [CrossRef]
- Ahmed, R.; Dunford, J.; Mehran, R.; Robson, S.; Kunadian, V. Pre-Eclampsia and Future Cardiovascular Risk among Women: A Review. J. Am. Coll. Cardiol. 2014, 63, 1815–1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, A.H.; Bushnell, C.D.; Jamison, M.G.; Myers, E.R. Incidence and Risk Factors for Stroke in Pregnancy and the Puerperium. Obstet. Gynecol. 2005, 106, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Ray, J.G.; Vermeulen, M.J.; Schull, M.J.; Redelmeier, D.A. Cardiovascular Health after Maternal Placental Syndromes (CHAMPS): Population-Based Retrospective Cohort Study. Lancet 2005, 366, 1797–1803. [Google Scholar] [CrossRef]
- Hooijschuur, M.C.E.; Ghossein-Doha, C.; Kroon, A.A.; De Leeuw, P.W.; Zandbergen, A.M.; Van Kuijk, S.M.J.; Spaanderman, M.E.A. Metabolic Syndrome and Pre-Eclampsia. Ultrasound Obstet. Gynecol. 2019, 54, 64–71. [Google Scholar] [CrossRef]
- Mosca, L.; Benjamin, E.J.; Berra, K.; Bezanson, J.L.; Dolor, R.J.; Lloyd-Jones, D.M.; Newby, L.K.; Piña, I.L.; Roger, V.L.; Shaw, L.J.; et al. American Heart Association. Effectiveness-Based Guidelines for the Prevention of Cardiovascular Disease in Women-2011 Update: A Guideline from the American Heart Association. J. Am. Coll. Cardiol. 2011, 57, 1404–1423. [Google Scholar] [CrossRef] [Green Version]
- Powe, C.E.; Levine, R.J.; Karumanchi, S.A. Preeclampsia, a Disease of the Maternal Endothelium: The Role of Antiangiogenic Factors and Implications for Later Cardiovascular Disease. Circulation 2011, 123, 2856–2869. [Google Scholar] [CrossRef]
- Smith, G.N.; Walker, M.C.; Liu, A.; Wen, S.W.; Swansburg, M.; Ramshaw, H.; White, R.R.; Roddy, M.; Hladunewich, M.; Pre-Eclampsia New Emerging Team (PE-NET). A History of Preeclampsia Identifies Women Who Have Underlying Cardiovascular Risk Factors. Am. J. Obstet. Gynecol. 2009, 200, 58. [Google Scholar] [CrossRef]
- Yang, J.J.; Lee, S.A.; Choi, J.Y.; Song, M.; Han, S.; Yoon, H.S.; Lee, Y.; Oh, J.; Lee, J.K.; Kang, D. Subsequent Risk of Metabolic Syndrome in Women with a History of Preeclampsia: Data from the Health Examinees Study. J. Epidemiol. 2015, 25, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Jenabi, E.; Afshari, M.; Khazaei, S. The Association between Preeclampsia and the Risk of Metabolic Syndrome after Delivery: A Meta-Analysis. J. Matern. Fetal Neonatal Med. 2021, 34, 3253–3258. [Google Scholar] [CrossRef]
- Smith, G.N.; Pudwell, J.; Walker, M.; Wen, S.W. Risk Estimation of Metabolic Syndrome at One and Three Years after a Pregnancy Complicated by Preeclampsia. J. Obstet. Gynaecol. Can. 2012, 34, 836–841. [Google Scholar] [CrossRef]
- Forest, J.C.; Girouard, J.; Massé, J.; Moutquin, J.M.; Kharfi, A.; Ness, R.B.; Roberts, J.M.; Giguère, Y. Early Occurrence of Metabolic Syndrome after Hypertension in Pregnancy. Obstet. Gynecol. 2005, 105, 1373–1380. [Google Scholar] [CrossRef]
- Cho, G.J.; Jung, U.S.; Sim, J.Y.; Lee, Y.J.; Bae, N.Y.; Choi, H.J.; Park, J.H.; Kim, H.J.; Oh, M.J. Is Preeclampsia Itself a Risk Factor for the Development of Metabolic Syndrome after Delivery? Obstet. Gynecol. Sci. 2019, 62, 233–241. [Google Scholar] [CrossRef]
- Veerbeek, J.H.W.; Hermes, W.; Breimer, A.Y.; van Rijn, B.B.; Koenen, S.V.; Mol, B.W.; Franx, A.; de Groot, C.J.M.; Koster, M.P.H. Cardiovascular Disease Risk Factors after Early-Onset Preeclampsia, Late-Onset Preeclampsia, and Pregnancy-Induced Hypertension. Hypertension 2015, 65, 600–606. [Google Scholar] [CrossRef] [Green Version]
- Stekkinger, E.; Zandstra, M.; Peeters, L.L.H.; Spaanderman, M.E.A. Early-Onset Preeclampsia and the Prevalence of Postpartum Metabolic Syndrome. Obstet. Gynecol. 2009, 114, 1076–1084. [Google Scholar] [CrossRef]
- Brantsæter, A.L.; Myhre, R.; Haugen, M.; Myking, S.; Sengpiel, V.; Magnus, P.; Jacobsson, B.; Meltzer, H.M. Intake of Probiotic Food and Risk of Preeclampsia in Primiparous Women. Am. J. Epidemiol. 2011, 174, 807–815. [Google Scholar] [CrossRef] [Green Version]
- Agerholm-Larsen, L.; Raben, A.; Haulrik, N.; Hansen, A.S.; Manders, M.; Astrup, A. Effect of 8 Week Intake of Probiotic Milk Products on Risk Factors for Cardiovascular Diseases. Eur. J. Clin. Nutr. 2000, 54, 288–297. [Google Scholar] [CrossRef]
- Aihara, K.; Kajimoto, O.; Hirata, H.; Takahashi, R.; Nakamura, Y. Effect of Powdered Fermented Milk with Lactobacillus Helveticus on Subjects with High-Normal Blood Pressure or Mild Hypertension. J. Am. Coll. Nutr. 2005, 24, 257–265. [Google Scholar] [CrossRef]
- Nordqvist, M.; Jacobsson, B.; Brantsæter, A.L.; Myhre, R.; Nilsson, S.; Sengpiel, V. Timing of Probiotic Milk Consumption during Pregnancy and Effects on the Incidence of Preeclampsia and Preterm Delivery: A Prospective Observational Cohort Study in Norway. BMJ Open 2018, 8, 18021. [Google Scholar] [CrossRef] [Green Version]
- Grev, J.; Berg, M.; Soll, R. Maternal Probiotic Supplementation for Prevention of Morbidity and Mortality in Preterm Infants. Cochrane Database Syst. Rev. 2018, 12, 12519. [Google Scholar] [CrossRef]
- Yeganegi, M.; Watson, C.S.; Martins, A.; Kim, S.O.; Reid, G.; Challis, J.R.G.; Bocking, A.D. Effect of Lactobacillus Rhamnosus GR-1 Supernatant and Fetal Sex on Lipopolysaccharide-Induced Cytokine and Prostaglandin-Regulating Enzymes in Human Placental Trophoblast Cells: Implications for Treatment of Bacterial Vaginosis and Prevention of Preterm Labor. Am. J. Obstet. Gynecol. 2009, 200, 532. [Google Scholar]
- Rahman, K.; Desai, C.; Iyer, S.S.; Thorn, N.E.; Kumar, P.; Liu, Y.; Smith, T.; Neish, A.S.; Li, H.; Tan, S.; et al. Loss of Junctional Adhesion Molecule A Promotes Severe Steatohepatitis in Mice on a Diet High in Saturated Fat, Fructose, and Cholesterol. Gastroenterology 2016, 151, 733–746. [Google Scholar] [CrossRef] [Green Version]
- Maslennikov, R.; Ivashkin, V.; Efremova, I.; Poluektova, E.; Shirokova, E. Gut-Liver Axis in Cirrhosis: Are Hemodynamic Changes a Missing Link? World J. Clin. Cases 2021, 9, 9320–9332. [Google Scholar] [CrossRef]
- Cope, K.; Risby, T.; Diehl, A.M. Increased Gastrointestinal Ethanol Production in Obese Mice: Implications for Fatty Liver Disease Pathogenesis. Gastroenterology 2000, 119, 1340–1347. [Google Scholar] [CrossRef]
- Salaspuro, M. Bacteriocolonic Pathway for Ethanol Oxidation: Characteristics and Implications. Ann. Med. 1996, 28, 195–200. [Google Scholar] [CrossRef]
- Nair, S.; Cope, K.; Risby, T.H.; Diehl, A.M.; Terence, R.H. Obesity and Female Gender Increase Breath Ethanol Concentration: Potential Implications for the Pathogenesis of Nonalcoholic Steatohepatitis. Am. J. Gastroenterol. 2001, 96, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- Aronsson, L.; Huang, Y.; Parini, P.; Korach-André, M.; Håkansson, J.; Gustafsson, J.Å.; Pettersson, S.; Arulampalam, V.; Rafter, J. Decreased Fat Storage by Lactobacillus Paracasei Is Associated with Increased Levels of Angiopoietin-Like 4 Protein (ANGPTL4). PLoS ONE 2010, 5, e13087. [Google Scholar] [CrossRef]
- Kadooka, Y.; Sato, M.; Ogawa, A.; Miyoshi, M.; Uenishi, H.; Ogawa, H.; Ikuyama, K.; Kagoshima, M.; Tsuchida, T. Effect of Lactobacillus Gasseri SBT2055 in Fermented Milk on Abdominal Adiposity in Adults in a Randomised Controlled Trial. Br. J. Nutr. 2013, 110, 1696–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiciński, M.; Gębalski, J.; Gołębiewski, J.; Malinowski, B. Probiotics for the Treatment of Overweight and Obesity in Humans—A Review of Clinical Trials. Microorganisms 2020, 8, 1148. [Google Scholar] [CrossRef]
- Ilmonen, J.; Isolauri, E.; Poussa, T.; Laitinen, K. Impact of Dietary Counselling and Probiotic Intervention on Maternal Anthropometric Measurements during and after Pregnancy: A Randomized Placebo-Controlled Trial. Clin. Nutr. 2011, 30, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Paek, K.; Lee, H.Y.; Park, J.H.; Lee, Y. Antiobesity Effect of Trans-10,Cis-12-Conjugated Linoleic Acid-Producing Lactobacillus Plantarum PL62 on Diet-Induced Obese Mice. J. Appl. Microbiol. 2007, 103, 1140–1146. [Google Scholar] [CrossRef]
- Kennedy, A.; Martinez, K.; Schmidt, S.; Mandrup, S.; LaPoint, K.; McIntosh, M. Antiobesity Mechanisms of Action of Conjugated Linoleic Acid. J. Nutr. Biochem. 2010, 21, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Bagarolli, R.A.; Tobar, N.; Oliveira, A.G.; Araújo, T.G.; Carvalho, B.M.; Rocha, G.Z.; Vecina, J.F.; Calisto, K.; Guadagnini, D.; Prada, P.O.; et al. Probiotics Modulate Gut Microbiota and Improve Insulin Sensitivity in DIO Mice. J. Nutr. Biochem. 2017, 50, 16–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational Diabetes Mellitus. Nat. Rev. Dis. Primers 2019, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Cano, P.G.; Santacruz, A.; Trejo, F.M.; Sanz, Y. Bifidobacterium CECT 7765 Improves Metabolic and Immunological Alterations Associated with Obesity in High-Fat Diet-Fed Mice. Obesity 2013, 21, 2310–2321. [Google Scholar] [CrossRef] [PubMed]
- Moya-Pérez, A.; Neef, A.; Sanz, Y. Bifidobacterium Pseudocatenulatum CECT 7765 Reduces Obesity-Associated Inflammation by Restoring the Lymphocyte-Macrophage Balance and Gut Microbiota Structure in High-Fat Diet-Fed Mice. PLoS ONE 2015, 10, e0126976. [Google Scholar] [CrossRef]
- Fawzy El-Sayed, K.M.; Klingebiel, P.; Dörfer, C.E. Toll-like Receptor Expression Profile of Human Dental Pulp Stem/Progenitor Cells. J. Endod. 2016, 42, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Cardona Gloria, Y.; Latz, E.; De Nardo, D. Generation of Innate Immune Reporter Cells Using Retroviral Transduction. Methods Mol. Biol. 2018, 1714, 97–117. [Google Scholar]
- Rosadini, C.V.; Kagan, J.C. Early Innate Immune Responses to Bacterial LPS. Curr. Opin. Immunol. 2017, 44, 14–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, I.; Yamanishi, S.; Cox, L.; Methé, B.A.; Zavadil, J.; Li, K.; Gao, Z.; Mahana, D.; Raju, K.; Teitler, I.; et al. Antibiotics in Early Life Alter the Murine Colonic Microbiome and Adiposity. Nature 2012, 488, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Martínez, I.; Wallace, G.; Zhang, C.; Legge, R.; Benson, A.K.; Carr, T.P.; Moriyama, E.N.; Walter, J. Diet-Induced Metabolic Improvements in a Hamster Model of Hypercholesterolemia Are Strongly Linked to Alterations of the Gut Microbiota. Appl. Environ. Microbiol. 2009, 75, 4175–4184. [Google Scholar] [CrossRef] [Green Version]
- Round, J.L.; Mazmanian, S.K. The Gut Microbiota Shapes Intestinal Immune Responses during Health and Disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef]
- Mazloom, K.; Siddiqi, I.; Covasa, M. Probiotics: How Effective Are They in the Fight against Obesity? Nutrients 2019, 11, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukdsi, M.C.A.; Cano, M.P.G.; González, S.N.; Medina, R.B. Administration of Lactobacillus Fermentum CRL1446 Increases Intestinal Feruloyl Esterase Activity in Mice. Lett. Appl. Microbiol. 2012, 54, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Fabersani, E.; Abeijón-Mukdsi, M.C.; Ross, R.; Fontana, C.; Benítez-Páez, A.; Gauffin-Cano, P.; Medina, R.B. Lactobacillus Fermentum CRL1446 Ameliorates Oxidative and Metabolic Parameters by Increasing Intestinal Feruloyl Esterase Activity and Modulating Microbiota in Caloric-Restricted Mice. Nutrients 2016, 8, 415. [Google Scholar] [CrossRef] [Green Version]
- Le Barz, M.; Daniel, N.; Varin, T.V.; Naimi, S.; Demers-Mathieu, V.; Pilon, G.; Audy, J.; Laurin, É.; Roy, D.; Urdaci, M.C.; et al. In Vivo Screening of Multiple Bacterial Strains Identifies Lactobacillus Rhamnosus Lb102 and Bifidobacterium Animalis Ssp. Lactis Bf141 as Probiotics That Improve Metabolic Disorders in a Mouse Model of Obesity. FASEB J. 2019, 33, 4921–4935. [Google Scholar] [CrossRef] [PubMed]
- Thiennimitr, P.; Yasom, S.; Tunapong, W.; Chunchai, T.; Wanchai, K.; Pongchaidecha, A.; Lungkaphin, A.; Sirilun, S.; Chaiyasut, C.; Chattipakorn, N.; et al. Lactobacillus Paracasei HII01, Xylooligosaccharides, and Synbiotics Reduce Gut Disturbance in Obese Rats. Nutrition 2018, 54, 40–47. [Google Scholar] [CrossRef]
- Borrelli, A.; Bonelli, P.; Tuccillo, F.M.; Goldfine, I.D.; Evans, J.L.; Buonaguro, F.M.; Mancini, A. Role of Gut Microbiota and Oxidative Stress in the Progression of Non-Alcoholic Fatty Liver Disease to Hepatocarcinoma: Current and Innovative Therapeutic Approaches. Redox Biol. 2018, 15, 467–479. [Google Scholar] [CrossRef]
- Brandi, G.; De Lorenzo, S.; Candela, M.; Pantaleo, M.A.; Bellentani, S.; Tovoli, F.; Saccoccio, G.; Biasco, G. Microbiota, NASH, HCC and the Potential Role of Probiotics. Carcinogenesis 2017, 38, 231–240. [Google Scholar] [CrossRef] [Green Version]
- Wigg, A.J.; Roberts-Thomson, I.C.; Dymock, R.B.; McCarthy, P.J.; Grose, R.H.; Cummins, A.G. The Role of Small Intestinal Bacterial Overgrowth, Intestinal Permeability, Endotoxaemia, and Tumour Necrosis Factor Alpha in the Pathogenesis of Non-Alcoholic Steatohepatitis. Gut 2001, 48, 206–211. [Google Scholar] [CrossRef] [Green Version]
- Miele, L.; Valenza, V.; La Torre, G.; Montalto, M.; Cammarota, G.; Ricci, R.; Mascianà, R.; Forgione, A.; Gabrieli, M.L.; Perotti, G.; et al. Increased Intestinal Permeability and Tight Junction Alterations in Nonalcoholic Fatty Liver Disease. Hepatology 2009, 49, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Boursier, J.; Mueller, O.; Barret, M.; Machado, M.; Fizanne, L.; Araujo-Perez, F.; Guy, C.D.; Seed, P.C.; Rawls, J.F.; David, L.A.; et al. The Severity of Nonalcoholic Fatty Liver Disease Is Associated with Gut Dysbiosis and Shift in the Metabolic Function of the Gut Microbiota. Hepatology 2016, 63, 764–775. [Google Scholar] [CrossRef] [Green Version]
- Caussy, C.; Hsu, C.; Lo, M.T.; Liu, A.; Bettencourt, R.; Ajmera, V.H.; Bassirian, S.; Hooker, J.; Sy, E.; Richards, L.; et al. Genetics of NAFLD in Twins Consortium. Link between Gut-Microbiome Derived Metabolite and Shared Gene-Effects with Hepatic Steatosis and Fibrosis in NAFLD. Hepatology 2018, 68, 918–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loomba, R.; Seguritan, V.; Li, W.; Long, T.; Klitgord, N.; Bhatt, A.; Dulai, P.S.; Caussy, C.; Bettencourt, R.; Highlander, S.K.; et al. Gut Microbiome-Based Metagenomic Signature for Non-Invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell Metab. 2017, 25, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.A.; Kim, J. Effect of Probiotics on Blood Lipid Concentrations: A Meta-Analysis of Randomized Controlled Trials. Medicine 2015, 94, 1714. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Hashiguchi, M.; Shiga, T.; Tamura, H.; Mochizuki, M. Meta-Analysis: Effects of Probiotic Supplementation on Lipid Profiles in Normal to Mildly Hypercholesterolemic Individuals. PLoS ONE 2015, 10, e139795. [Google Scholar]
- Companys, J.; Pla-Pagà, L.; Calderón-Pérez, L.; Llauradó, E.; Solà, R.; Pedret, A.; Valls, R.M. Fermented Dairy Products, Probiotic Supplementation, and Cardiometabolic Diseases: A Systematic Review and Meta-Analysis. Adv. Nutr. 2020, 11, 834–863. [Google Scholar] [CrossRef] [PubMed]
- Speiser, P.W.; Rudolf, M.C.J.; Anhalt, H.; Camacho-Hubner, C.; Chiarelli, F.; Eliakim, A.; Freemark, M.; Gruters, A.; Hershkovitz, E.; Iughetti, L.; et al. Childhood Obesity. J. Clin. Endocrinol. Metab. 2005, 90, 1871–1887. [Google Scholar] [CrossRef] [PubMed]
- Matteoni, C.A.; Younossi, Z.M.; Gramlich, T.; Boparai, N.; Liu, Y.C.; McCullough, A.J. Nonalcoholic Fatty Liver Disease: A Spectrum of Clinical and Pathological Severity. Gastroenterology 1999, 116, 1413–1419. [Google Scholar] [CrossRef]
- Mandato, C.; Lucariello, S.; Licenziati, M.R.; Franzese, A.; Spagnuolo, M.I.; Ficarella, R.; Pacilio, M.; Amitrano, M.; Capuano, G.; Meli, R.; et al. Metabolic, Hormonal, Oxidative, and Inflammatory Factors in Pediatric Obesity-Related Liver Disease. J. Pediatr. 2005, 147, 62–66. [Google Scholar] [CrossRef]
- Schwimmer, J.B.; Zepeda, A.; Newton, K.P.; Xanthakos, S.A.; Behling, C.; Hallinan, E.K.; Donithan, M.; Tonascia, J.; Nonalcoholic Steatohepatitis Clinical Research Network. Longitudinal Assessment of High Blood Pressure in Children with Nonalcoholic Fatty Liver Disease. PLoS ONE 2014, 9, e112569. [Google Scholar] [CrossRef]
- Newton, K.P.; Hou, J.; Crimmins, N.A.; Lavine, J.E.; Barlow, S.E.; Xanthakos, S.A.; Africa, J.; Behling, C.; Donithan, M.; Clark, J.M.; et al. Nonalcoholic Steatohepatitis Clinical Research Network. Prevalence of Prediabetes and Type 2 Diabetes in Children with Nonalcoholic Fatty Liver Disease. JAMA Pediatr. 2016, 170, 161971. [Google Scholar] [CrossRef] [PubMed]
- Schwimmer, J.B.; Deutsch, R.; Rauch, J.B.; Behling, C.; Newbury, R.; Lavine, J.E. Obesity, Insulin Resistance, and Other Clinicopathological Correlates of Pediatric Nonalcoholic Fatty Liver Disease. J. Pediatr. 2003, 143, 500–505. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rastelli, M.; Knauf, C.; Cani, P.D. Gut Microbes and Health: A Focus on the Mechanisms Linking Microbes, Obesity, and Related Disorders. Obesity 2018, 26, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, R.; Clarkson, V.; Verdonk, R.C.; Marais, A.D.; Shephard, E.G.; Ryffel, B.; Hall, P. Rodent Nutritional Model of Steatohepatitis: Effects of Endotoxin (Lipopolysaccharide) and Tumor Necrosis Factor Alpha Deficiency. J. Gastroenterol. Hepatol. 2006, 21, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.; Fabersani, E.; Marquez, A.; Gauffin-Cano, P. Adipose Tissue Inflammation and Metabolic Syndrome. The Proactive Role of Probiotics. Eur. J. Nutr. 2019, 58, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Clarke, G.; Cryan, J.F.; Dinan, T.G. Brain-Gut-Microbiota Axis: Challenges for Translation in Psychiatry. Ann. Epidemiol. 2016, 26, 366–372. [Google Scholar] [CrossRef]
- Kemgang, T.; Kapila, S.; Shanmugam, V.; Kapila, R. Cross-Talk between Probiotic Lactobacilli and Host Immune System. J. Appl. Microbiol. 2014, 117, 303–319. [Google Scholar] [CrossRef]
- Zhang, L.; Li, N.; Caicedo, R.; Neu, J. Alive and Dead Lactobacillus Rhamnosus GG Decrease Tumor Necrosis Factor-α–Induced Interleukin-8 Production in Caco-2 Cells. J. Nutr. 2005, 135, 1752–1756. [Google Scholar] [CrossRef] [PubMed]
- Tien, M.T.; Girardin, S.E.; Regnault, B.; Bourhis, L.L.; Dillies, M.A.; Coppée, J.Y.; Bourdet-Sicard, R.; Sansonetti, P.J.; Pédron, T. Anti-Inflammatory Effect of Lactobacillus Casei on Shigella-Infected Human Intestinal Epithelial Cells. J. Immunol. 2006, 176, 1228–1237. [Google Scholar] [CrossRef] [Green Version]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and Functional Importance in the Gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Rossi, P.; Difrancia, R.; Quagliariello, V.; Savino, E.; Tralongo, P.; Randazzo, C.; Berretta, M. B-glucans from Grifola frondosa and Ganoderma lucidum in breast cancer: An example of complementary and integrative medicine. Oncotarget 2018, 9, 24837–24856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yucel, C.; Quagliariello, V.; Iaffaioli, R.; Ferrari, G.; Donsì, F. Submicron complex lipid carriers for curcumin delivery to intestinal epithelial cells: Effect of different emulsifiers on bioaccessibility and cell uptake. Int. J. Pharm. 2015, 15, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Berretta, M.; Quagliariello, V.; Maurea, N.; Di Francia, R.; Sharifi, S.; Facchini, G.; Rinaldi, L.; Piezzo, M.; Manuela, C.; Nunnari, G.; et al. Multiple Effects of Ascorbic Acid against Chronic Diseases: Updated Evidence from Preclinical and Clinical Studies. Antioxidants 2020, 9, 1182. [Google Scholar] [CrossRef] [PubMed]

| Measure | NCEP ATP3 2005 | IDF 2009 |
|---|---|---|
| Elevated waist circumference | ≥88 cm (≥34.6 inches) | ≥80 cm (≥31.5 inches) |
| Elevated triglycerides (TG) | ≥150 mg/dL (1.7 mmol/L) or drug treatment for elevated TG | ≥150 mg/dL (1.7 mmol/L) or drug treatment for high TG |
| Reduced high-density lipoprotein (HDL) cholesterol | <50 mg/dL (1.3 mmol/L) or drug treatment for low HDL cholesterol | <50 mg/dL (1.3 mmol/L) or drug treatment for low HDL cholesterol |
| Elevated blood pressure (BP) | ≥130 mmHg systolic BP or ≥85 mmHg diastolic BP or drug treatment for hypertension | ≥130 mmHg systolic BP or ≥85 mmHg diastolic BP or drug treatment for hypertension |
| Elevated fasting glucose | ≥100 mg/dL (≥5.6 mmol/L) or drug treatment for elevated blood glucose | ≥100 mg/dL (≥5.6 mmol/L) or diagnosed diabetes |
| Reference | Strain | Dosage | Treatment Duration | Population | Results |
|---|---|---|---|---|---|
| Kijmanawat et al. (2019) [7] | Lactobacillus acidophilus and Bifidobacterium bifidum | 109 CFU/capsule | 4 weeks | 30 patients with GDM |
|
| Nabhani et al. (2018) [111] | Synbiotic capsule consisting of Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus fermentum, Lactobacillus gasseri with fructooligosaccharide (38.5 mg) | 1.5–7.0 × 109–10 CFU/g | 6 weeks | 45 patients with GDM |
|
| Sahhaf Ebrahimi et al. (2019) [112] | Lactobacillus acidophilus and Bifidobacterium lactis | 106 (300 mg of probiotic yoghurt) | 8 weeks | 42 patients with GDM |
|
| Babadi et al. (2019) [114] | Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum and Lactobacillus fermentum | 2 × 109 CFU/g | 6 weeks | 24 patients with GDM |
|
| Pellonperä et al. (2019) [119] | Lactobacillus rhamnosus and Bifidobacterium animalis ssp. lactis | 1010 CFU/capsule | throughout the pregnancy, and until 6 months postpartum | 439 overweight or obese pregnant women |
|
| Callaway et al. (2019) [120] | Lactobacillus rhamnosus and Bifidobacterium animalis subspecies lactis | 109 CFU/capsule | throughout pregnancy from the first half of the second trimester | 207 overweight and obese women prevent GDM |
|
| Badehnoosh et al. (2018) [134] | Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium bifidum | 2 × 109 CFU/g | 6 weeks | 60 patients with GDM |
|
| Okesene-Gafa et al. (2019) [150] | Lactobacillus rhamnosus GG and Bifidobacterium lactis BB12 | 6.5 × 109 CFU/capsule | throughout the pregnancy | 230 obese pregnant women |
|
| Brantsæter et al. (2011) [175] | Lactobacillus acidophilus, Bifidobacterium lactis and Lactobacillus rhamnosus | 108 CFU/mL | the first halfof pregnancy | 33,399 primiparous women |
|
| Nordqvist et. al. (2018) [178] | Lactobacillus acidophilus, Bifidobacterium lactis and Lactobacillus rhamnosus | 108 CFU/mL | Early pregnancy or late pregnancy | 37,050 primiparous women |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obuchowska, A.; Gorczyca, K.; Standyło, A.; Obuchowska, K.; Kimber-Trojnar, Ż.; Wierzchowska-Opoka, M.; Leszczyńska-Gorzelak, B. Effects of Probiotic Supplementation during Pregnancy on the Future Maternal Risk of Metabolic Syndrome. Int. J. Mol. Sci. 2022, 23, 8253. https://doi.org/10.3390/ijms23158253
Obuchowska A, Gorczyca K, Standyło A, Obuchowska K, Kimber-Trojnar Ż, Wierzchowska-Opoka M, Leszczyńska-Gorzelak B. Effects of Probiotic Supplementation during Pregnancy on the Future Maternal Risk of Metabolic Syndrome. International Journal of Molecular Sciences. 2022; 23(15):8253. https://doi.org/10.3390/ijms23158253
Chicago/Turabian StyleObuchowska, Aleksandra, Kamila Gorczyca, Arkadiusz Standyło, Karolina Obuchowska, Żaneta Kimber-Trojnar, Magdalena Wierzchowska-Opoka, and Bożena Leszczyńska-Gorzelak. 2022. "Effects of Probiotic Supplementation during Pregnancy on the Future Maternal Risk of Metabolic Syndrome" International Journal of Molecular Sciences 23, no. 15: 8253. https://doi.org/10.3390/ijms23158253