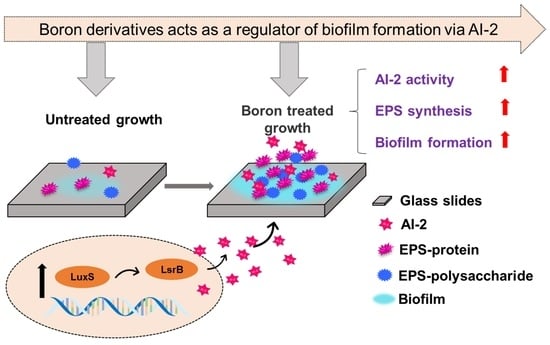
Boron Derivatives Accelerate Biofilm Formation of Recombinant Escherichia coli via Increasing Quorum Sensing System Autoinducer-2 Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of Boron Derivatives on the AI-2 Activity of Recombinant E. coli
2.2. Growth Analysis of Biofilm after the Adding of Boron Derivatives
2.3. Biofilm Assay by SEM and CLSM
2.4. Effect of Adding Boron Derivatives on the EPS of Recombinant E. coli
2.5. Linear Correlation between the Activity of Signal Molecule AI-2 and EPS
2.6. Transcription Level of Biofilm Formation, QS and Flagellar Movement Related Genes
2.7. Boron Enhances Biofilm Formation to Affect Enzymatic Catalysis
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Bacterial Strains and Culture Conditions
3.3. Effects of Different Boron Derivatives on the AI-2 Activity of Recombinant E. coli
3.4. Total Biomass Assay of Recombinant E. coli Biofilm
3.5. Extraction of EPS
3.6. RNA Isolation and qRT-PCR
3.7. Visualization of the Biofilms Using CLSM and SEM
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nguyen, T.D.P.; Vo, C.T.; Nguyen-Sy, T.; Tran, T.N.T.; Le, T.V.A.; Chiu, C.; Sankaran, R.; Show, P.L. Utilization of microalgae for self-regulation of extracellular polymeric substance production. Biochem. Eng. J. 2020, 159, 107616. [Google Scholar] [CrossRef]
- Rosche, B.; Li, X.Z.; Hauer, B.; Schmid, A.; Buehler, K. Microbial biofilms: A concept for industrial catalysis? Trends Biotechnol. 2009, 27, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Gross, R.; Hauer, B.; Otto, K.; Schmid, A. Microbial biofilms: New catalysts for maximizing productivity of long-term biotransformations. Biotechnol. Bioeng. 2010, 98, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, J.M.; Rai, A.K.; Sharma, M.; Tripathi, M.; Prasad, R. Microbial biofilms: Recent advances and progress in environmental bioremediation. Sci. Total Environ. 2022, 824, 153843. [Google Scholar] [CrossRef] [PubMed]
- Deena, S.R.; Kumar, G.; Vickram, A.S.; Singhania, R.R.; Dong, C.D.; Rohini, K.; Anbarasu, K.; Thanigaivel, S.; Ponnusamy, V.K. Efficiency of various biofilm carriers and microbial interactions with substrate in moving bed-biofilm reactor for environmental wastewater treatment. Bioresour. Technol. 2022, 359, 127421. [Google Scholar] [CrossRef]
- Gross, R.; Lang, K.; Bühler, K.; Schmid, A. Characterization of a biofilm membrane reactor and its prospects for fine chemical synthesis. Biotechnol. Bioeng. 2010, 105, 705–717. [Google Scholar] [CrossRef]
- Ghosh, S.; Nag, M.; Lahiri, D.; Sarkar, T.; Pati, S.; Kari, Z.A.; Nirmal, N.P.; Edinur, H.A.; Ray, R.R. Engineered Biofilm: Innovative Nextgen Strategy for Quality Enhancement of Fermented Foods. Front. Nutr. 2022, 9, 808630. [Google Scholar] [CrossRef]
- Al-Kaidy, H.; Duwe, A.; Huster, M.; Muffler, K.; Schlegel, C.; Sieker, T.; Stadtmüller, R.; Tippkötter, N.; Ulber, R. Biotechnology and Bioprocess Engineering—From the First Ullmanns Article to Recent Trends. Chem. Ing. Tech. 2014, 86, 2215–2225. [Google Scholar] [CrossRef]
- Emerenini, B.O.; Eberl, H.J. Reactor scale modeling of quorum sensing induced biofilm dispersal. Appl. Math. Comput. 2022, 418, 126792. [Google Scholar] [CrossRef]
- Ha, J.; Hauk, P.; Cho, K.; Eo, Y.; Ma, X.; Stephens, K.; Cha, S.; Jeong, M.; Suh, J.; Sintim, H.O.; et al. Evidence of link between quorum sensing and sugar metabolism in Escherichia coli revealed via cocrystal structures of LsrK and HPr. Sci. Adv. 2018, 4, eaar7063. [Google Scholar] [CrossRef] [Green Version]
- Xuan, G.H.; Lin, H.; Tan, L.; Zhao, G.; Wang, J.X. Quorum Sensing Promotes Phage Infection in Pseudomonas aeruginosa PAO1. mBio 2022, 13, e0317421. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.; Hooshangi, S.; Wu, H.; Valdes, J.J.; Bentley, W.E. Autonomous induction of recombinant proteins by minimally rewiring native quorum sensing regulon of E. coli. Metab. Eng. 2010, 12, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.C.; Sun, Q.; Fu, C.Y.; She, X.; Liu, X.C.; He, Y.; Ai, Q.; Li, L.Q.; Wang, Z.L. Exogenous autoinducer-2 rescues intestinal dysbiosis and intestinal inflammation in a neonatal mouse necrotizing enterocolitis model. Front. Cell. Infect. Microbiol. 2021, 11, 694395. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.H.; Liu, Y. Involvement of ATP and autoinducer-2 in aerobic granulation. Biotechnol. Bioeng. 2010, 105, 51–58. [Google Scholar] [CrossRef]
- Ren, T.T.; Yu, H.Q.; Li, X.Y. The quorum-sensing effect of aerobic granules on bacterial adhesion, biofilm formation, and sludge granulation. Appl. Microbiol. Biot. 2010, 88, 789–797. [Google Scholar] [CrossRef] [Green Version]
- Coulthurst, S.J.; Whitehead, N.A.; Welch, M.; Salmond, G.P.C. Can boron get bacteria talking? Trends Biochem. Sci. 2002, 27, 217–219. [Google Scholar] [CrossRef]
- Chen, X.; Schauder, S.; Potier, N.; Alain, V.D.; István, P.; Bassler, B.L.; Hughson, F.M. Structural identification of a bacterial quorum-sensing signal containing boron. Nature 2002, 415, 545–549. [Google Scholar] [CrossRef]
- Semmelhack, M.F.; Campagna, S.R.; Hwa, C.; Federle, M.J.; Bassler, B.L. Boron binding with the quorum sensing signal AI-2 and analogues. Org. Lett. 2004, 6, 2635–2637. [Google Scholar] [CrossRef]
- Zhang, S.H.; Yu, X.; Guo, F.; Wu, Z.Y. Effect of interspecies quorum sensing on the formation of aerobic granular sludge. Water Sci. Technol. 2011, 64, 1284–1290. [Google Scholar] [CrossRef]
- Xu, Z.Q.; Yang, Q.L.; Zhu, Y.L. Transcriptome analysis reveals the molecular mechanisms of the novel Lactobacillus pentosus pentocin against Bacillus cereus. Food Res. Int. 2022, 151, 110840. [Google Scholar] [CrossRef]
- Semmelhack, M.F.; Campagna, S.R.; Federle, M.J.; Bassler, B.L. An expeditious synthesis of DPD and boron binding studies. Org. Lett. 2005, 7, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Mondal, N.; Ghosh, W.; Chakraborty, R. Inducible boron resistance via active efflux in Lysinibacillus and Enterococcus isolates from boron-contaminated agricultural soil. Biometals 2022, 35, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Capalash, N.; Sharma, P. Expression of Meiothermus ruber luxS in E. coli alters the antibiotic susceptibility and biofilm formation. Appl. Microbiol. Biot. 2020, 104, 4457–4469. [Google Scholar] [CrossRef]
- Bassler, B.L.; Wright, M.; Showalter, R.E.; Silverman, M.R. Intercellular signalling in Vibrio harveyi: Sequence and function of genes regulating expression of luminescence. Mol. Microbiol. 2010, 9, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Dotto, C.; Serrat, A.L.; Ledesma, M.; Vay, C.; Ehling-Schulz, M.; Sordelli, D.O.; Grunert, T.; Buzzola, F. Salicylic acid stabilizes Staphylococcus aureus biofilm by impairing the agr quorum-sensing system. Sci. Rep. 2021, 11, 2953. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Olivier, H. Biofilm research within irrigation water distribution systems: Trends, knowledge gaps, and future perspectives. Sci. Total Environ. 2019, 673, 254–265. [Google Scholar] [CrossRef]
- Goldbach, H.E.; Wimmer, M.A. Boron in plants and animals: Is there a role beyond cell-wall structure? J. Plant Nutr. Soil Sci. 2007, 170, 39–48. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, L.; Zhang, Z.C.; Fan, H.J.; Cheng, X.C.; Lu, C.P. Biofilm formation, host-cell adherence, and virulence genes regulation of Streptococcus suis in response to autoinducer-2 signaling. Curr. Microbiol. 2014, 68, 575–580. [Google Scholar] [CrossRef]
- Beyenal, H.; Lewandowski, Z.; Harkin, G. Quantifying biofilm structure: Facts and fiction. Biofouling 2004, 20, 1–23. [Google Scholar] [CrossRef]
- Janjaroen, D.; Ling, F.Q.; Monroy, G.; Derlon, N.; Mogenroth, E.; Boppart, S.A.; Liu, W.T.; Nguyen, T.H. Roles of ionic strength and biofilm roughness on adhesion kinetics of Escherichia coli onto groundwater biofilm grown on PVC surfaces. Water Res. 2013, 47, 2531–2542. [Google Scholar] [CrossRef] [Green Version]
- E, J.J.; Ma, R.Z.; Chen, Z.C.; Yao, C.Q.; Wang, R.X.; Zhang, Q.L.; He, Z.B.; Sun, R.Y.; Wang, J.G. Improving the freeze-drying survival rate of Lactobacillus plantarum LIP-1 by increasing biofilm formation based on adjusting the composition of buffer salts in medium. Food Chem. 2020, 388, 128134. [Google Scholar] [CrossRef]
- Jiang, W.; Xia, S.Q.; Liang, J.; Zhang, Z.Q.; Hermanowicz, S.W. Effect of quorum quenching on the reactor performance, biofouling and biomass characteristics in membrane bioreactors. Water Res. 2013, 47, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.H.; Gu, Y.L.; Zeng, G.M.; Yang, Y.; Ouyang, Y.C.; Shi, L.X.; Shi, Y.H.; Yi, K.X. Control of indigenous quorum quenching bacteria on membrane biofouling in a short-period MBR. Bioresour. Technol. 2019, 283, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Kunacheva, C.; Stuckey, D.C. Analytical methods for soluble microbial products (SMP) and extracellular polymers (ECP) in wastewater treatment systems: A review. Water Res. 2014, 61, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.P.; Yu, H.Q.; Li, X.Y. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef]
- Nahm, C.H.; Kim, K.; Min, S.; Lee, H.; Chae, D.; Lee, K.; Choo, K.H.; Lee, C.H.; Koyuncu, I.; Park, P.K. Quorum sensing: An emerging link between temperature and membrane biofouling in membrane bioreactors. Biofouling 2019, 35, 443–453. [Google Scholar] [CrossRef]
- Keiski, C.L.; Harwich, M.; Jain, S.; Neculai, A.M.; Yip, P.; Robinson, H.; Whitney, J.C.; Riley, L.; Burrows, L.L.; Ohman, D.E.; et al. AlgK is a TPR-containing protein and the periplasmic component of a novel exopolysaccharide secretin. Structure 2010, 18, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Shiba, Y.; Miyagawa, H.; Nagahama, H.; Matsumoto, K.; Kondo, D.; Matsuoka, S.; Matsumoto, K.; Hara, H. Exploring the relationship between lipoprotein mislocalization and activation of the Rcs signal transduction system in Escherichia coli. Microbiology 2012, 158, 1238–1248. [Google Scholar] [CrossRef] [Green Version]
- Herzog, R.; Peschek, N.; Frohlich, K.S.; Schumacher, K.; Papenfort, K. Three autoinducer molecules act in concert to control virulence gene expression in Vibrio cholerae. Nucleic Acids Res. 2019, 47, 3171–3183. [Google Scholar] [CrossRef] [Green Version]
- Guo, M.H.; Fang, Z.J.; Sun, L.; Sun, D.F.; Wang, Y.L.; Li, C.; Wang, R.D.; Liu, Y.; Hu, H.Q.; Liu, Y.; et al. Regulation of thermostable direct hemolysin and biofilm formation of Vibrio parahaemolyticus by Quorum-Sensing genes luxM and luxS. Curr. Microbiol. 2018, 75, 1190–1197. [Google Scholar] [CrossRef]
- Simm, R.; Remminghorst, U.; Ahmad, I.; Zakikhany, K.; Romling, U. A role for the EAL-like protein STM1344 in regulation of CsgD expression and motility in Salmonella enterica serovar Typhimurium. J. Bacteriol. 2009, 191, 3928–3937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weir, E.K.; Martin, L.C.; Poppe, C.; Coombes, B.K.; Boerlin, P. Subinhibitory concentrations of tetracycline affect virulence gene expression in a multi-resistant Salmonella enterica subsp. enterica serovar Typhimurium DT104. Microbes Infect. 2008, 10, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Galdino, R.V.; Benevides, C.A.; Tenório, R.P. Diffusion maps of Bacillus subtilis biofilms via magnetic resonance imaging highlight a complex network of channels. Colloid Surf. B 2020, 190, 110905. [Google Scholar] [CrossRef]
- Wang, F.Q.; He, S.; Zhu, C.T.; Rabausch, U.; Streit, W.; Wang, J. The combined use of a continuous-flow microchannel reactor and ionic liquid cosolvent for efficient biocatalysis of unpurified recombinant enzyme. J. Chem. Technol. Biot. 2018, 93, 2671–2680. [Google Scholar] [CrossRef]
- Surette, M.G.; Miller, M.B.; Bassler, B.L. Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: A new family of genes responsible for autoinducer production. Proc. Natl. Acad. Sci. USA 1999, 96, 1639–1644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Toole, G.A.; Kolter, R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: A genetic analysis. Mol. Microbiol. 2010, 28, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, 45. [Google Scholar] [CrossRef]
- Qian, H.; Li, W.; Guo, L.X.; Tan, L.; Liu, H.Q.; Wang, J.J.; Pan, Y.J.; Zhao, Y. Stress response of Vibrio parahaemolyticus and Listeria monocytogenes biofilms to different modified atmospheres. Front. Microbiol. 2020, 11, 23. [Google Scholar] [CrossRef] [Green Version]









| Treatment Group | Sp (μm) | Sv (μm) | Sz (μm) | Average Thickness (μm) |
|---|---|---|---|---|
| Control | 7.16 | 5.84 | 13.00 | 4.80 ± 0.78 c |
| Na2B4O7 | 8.61 | 9.30 | 17.91 | 5.63 ± 0.28 c |
| NaBO3 | 7.49 | 8.15 | 15.64 | 5.66 ± 0.75 c |
| NaBO2 | 10.23 | 13.59 | 23.82 | 8.11 ± 0.85 b |
| H3BO3 | 14.06 | 15.86 | 29.91 | 11.13 ± 1.17 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Yan, C.-H.; Zhan, Y.-F.; Geng, L.-T.; Zhu, L.-L.; Gong, L.-C.; Wang, J. Boron Derivatives Accelerate Biofilm Formation of Recombinant Escherichia coli via Increasing Quorum Sensing System Autoinducer-2 Activity. Int. J. Mol. Sci. 2022, 23, 8059. https://doi.org/10.3390/ijms23158059
Chen H, Yan C-H, Zhan Y-F, Geng L-T, Zhu L-L, Gong L-C, Wang J. Boron Derivatives Accelerate Biofilm Formation of Recombinant Escherichia coli via Increasing Quorum Sensing System Autoinducer-2 Activity. International Journal of Molecular Sciences. 2022; 23(15):8059. https://doi.org/10.3390/ijms23158059
Chicago/Turabian StyleChen, Huan, Cheng-Hai Yan, Yu-Fan Zhan, Li-Tian Geng, Lin-Lin Zhu, Lu-Chan Gong, and Jun Wang. 2022. "Boron Derivatives Accelerate Biofilm Formation of Recombinant Escherichia coli via Increasing Quorum Sensing System Autoinducer-2 Activity" International Journal of Molecular Sciences 23, no. 15: 8059. https://doi.org/10.3390/ijms23158059