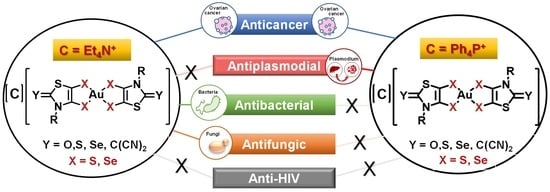
Broad Spectrum Functional Activity of Structurally Related Monoanionic Au(III) Bis(Dithiolene) Complexes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Redox Properties of the Gold Complexes
2.2. Molecular Structure and Intermolecular Interactions in the Solid State
2.3. Cytotoxicity in Ovarian Cells
2.4. Toxicity Studies in Zebrafish Embryo
2.5. Antiplasmodial Activity
2.6. Antibacterial and Antifungal Activity
2.7. Cytotoxicity and Anti-HIV Activity of Auranofin, [P][AuSEt(=S)] and [P][AuSBu(=S)]
2.8. Mechanistic Studies
2.8.1. Inhibition of TrxR by the Gold Complexes
2.8.2. DNA Electrophoresis
2.8.3. Gold-Complexes–HSA Interaction Studies
3. Materials and Methods
3.1. General Methods for Chemistry
3.2. General Synthetic Procedures
3.2.1. Typical Procedure for the Synthesis of the Dithiolene and Diselenolene Proligand 2-XR(=Y):
3.2.2. Procedure for the Synthesis of Monoanionic Gold Bis(Dithiolene) and Bis Diselenolene Complexes:
3.3. Crystallography
3.4. Biological Studies
3.4.1. Cytotoxic Activity
3.4.2. Toxicological Assessment in Zebrafish Embryo
3.4.3. In Vitro Activity of Gold Complexes against the Hepatic Stage of P. berghei Infection
3.4.4. In Vitro Activity of Gold Complexes against the Blood Stage of P. falciparum Infection
3.4.5. Bacterial and Fungal Strains
3.4.6. Antimicrobial Activity
3.4.7. Anti-HIV Assays
3.5. Mechanistic Studies for Relevant Complexes
3.5.1. DNA
3.5.2. Thioredoxin (TrxR) Inhibition Study
3.5.3. HSA-Binding Experiments
Sample Preparation for Spectrofluorometric Experiments
Fluorescence Spectroscopic Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Paprochcka, R.; Wiese-Szadkowska, M.; Janciauskiene, S.; Kosmalski, T.; Kulik, M.; Helmin-Basa, A. Latest developments in metal complexes as anticancer agents. Coord. Chem. Rev. 2022, 452, 214307. [Google Scholar] [CrossRef]
- Zhang, P.; Sadler, P.J. Redox-active metal complexes for anticancer therapy. Eur. J. Inorg. Chem. 2017, 12, 1541–1548. [Google Scholar] [CrossRef] [Green Version]
- Frei, A.; Zuegg, J.; Elliott, A.G.; Baker, M.; Braese, S.; Brown, C.; Chen, F.; Dowson, C.G.; Dujardin, G.; Jung, N.; et al. Metal complexes as a promising source for new antibiotics. Chem. Sci. 2020, 11, 2627–2639. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.-Y.; Shen, Q.-H.; Mao, Z.-W.; Tan, C.-P. Rising interest in the development of metal complexes in cancer immunotherapy. Chem. Asian J. 2022, 2022, e202200270. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.; Roy, P.K.; Ali, R.; Zakaria, C.; Zahan, K.-E. Selected pharmacological applications of 1st row transition metal complexes: A review. Clin. Med. Res. 2017, 6, 177–191. [Google Scholar] [CrossRef] [Green Version]
- Anthony, E.J.; Bolitho, E.M.; Bridgewater, H.E.; Carter, O.W.L.; Donnelly, J.M.; Imberti, C.; Lant, R.C.; Lermyte, F.; Needham, R.J.; Palau, M.; et al. Metallodrugs are unique: Opportunities and challenges of discovery and development. Chem. Sci. 2020, 11, 12888–12917. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, J.T.; Lipp, H.-P. Toxicity of platinum compounds. Expert Opin. Pharmacother. 2003, 4, 889–901. [Google Scholar] [CrossRef]
- Hanif, M.; Hartinger, C.G. Anticancer metallodrugs: Where is the next cisplatin? Future Med. Chem. 2018, 10, 615–617. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Nardon, C.; Boscutti, G.; Fregona, D. Beyond platinums: Gold complexes as anticancer agents. Anticancer Res. 2014, 34, 487–492. [Google Scholar] [PubMed]
- Kim, J.H.; Reeder, R.; Parkin, S.; Awuah, S.G. Gold(I/III)-phosphine complexes as potent antiproliferative agents. Sci. Rep. 2019, 9, 12335. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Wei, S.; Kou, P. Current progress and perspectives on using gold compounds for the modulation of tumor cell metabolism. Front. Chem. 2021, 9, 733463. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, B.; Williams, M.R.M.; Bochmann, M. Gold(III) complexes for antitumor applications: An overview. Chem. Eur. J. 2018, 24, 11840. [Google Scholar] [CrossRef]
- Radisavljević, S.; Bratsos, I.; Scheurer, A.; Korzekwa, J.; Masnikosa, R.; Tot, A.; Masnikosa, R.; Gligorijević, N.; Radulović, S.; Simović, A.R. New gold pincer-type complexes: Synthesis, characterization, DNA binding studies and cytotoxicity. Dalton Trans. 2018, 47, 13696. [Google Scholar] [CrossRef]
- Bertrand, B.; Casini, A. A golden future in medicinal inorganic chemistry: The promise of anticancer gold organometallic compounds. Dalton Trans. 2014, 3, 4209–4219. [Google Scholar] [CrossRef]
- Fung, S.K.; Zou, T.; Cao, B.; Lee, P.-Y.; Fung, Y.M.E.; Hu, D.; Lok, C.-N.; Che, C.-M. Cyclometalated gold(III) complexes containing N-heterocyclic carbene ligands engage multiple anti-cancer molecular targets. Angew. Chem. Int. Ed. 2017, 56, 3892–3896. [Google Scholar] [CrossRef]
- Carboni, S.; Zucca, A.; Stoccoro, S.; Maiore, L.; Arca, M.; Ortu, F.; Artner, C.; Keppler, B.K.; Meier-Menches, S.M.; Casini, A.; et al. New variations on the theme of gold(III) C∧N∧N cyclometalated complexes as anticancer agents: Synthesis and biological characterization. Inorg. Chem. 2018, 57, 14852–14865. [Google Scholar] [CrossRef] [Green Version]
- Magherini, F.; Fiaschi, T.; Valocchia, E.; Becatti, M.; Pratesi, A.; Marzo, T.; Massai, L.; Gabbiani, C.; Landini, I.; Nobili, S.; et al. Antiproliferative effects of two gold(I)-N-heterocyclicarbene complexes in A2780 human ovarian cancer cells: A comparative proteomic study. Oncotarget 2018, 9, 28042–28068. [Google Scholar] [CrossRef]
- Liu, W.; Gust, R. Metal N-heterocyclic carbene complexes as potential antitumor metallodrugs. Chem. Soc. Rev. 2013, 42, 755–773. [Google Scholar] [CrossRef]
- Ssemaganda, A.; Low, L.M.; Verhoeft, K.R.; Wambuzi, M.; Kawoozo, B.; Nabasumba, S.B.; Mpendo, J.; Bagaya, B.S.; Kiwanuka, N.; Stanisic, D.I.; et al. Good, Gold(I) phosphine compounds as parasite attenuating agents for malaria vaccine and drug development. Metallomics 2018, 10, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Dominelli, B.; Correia, J.D.G.; Kühn, F.E. Medicinal Applications of Gold(I/III)-Based Complexes Bearing N-Heterocyclic Carbene and Phosphine Ligands. J. Organomet. Chem. 2018, 866, 153–164. [Google Scholar] [CrossRef]
- Mora, M.; Gimeno, M.C.; Visbal, R. Recent advances in gold—NHC complexes with biological properties. Chem. Soc. Rev. 2019, 48, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Gust, R. Update on metal N-heterocyclic carbene complexes as potential anti-tumor metallodrugs. Coord. Chem. Rev. 2016, 329, 191–213. [Google Scholar] [CrossRef]
- Goetzfried, S.K.; Kapitza, P.; Gallati, C.M.; Nindl, A.; Cziferszky, M.; Hermann, M.; Wurst, K.; Kircher, B.; Gust, R. Investigations of the reactivity, stability and biological activity of halido (NHC)gold(I) complexes. Dalton Trans. 2022, 51, 1395–1406. [Google Scholar] [CrossRef]
- Yeo, C.I.; Ooi, K.K.; Tiekink, E.R.T. Gold-Based Medicine: A Paradigm Shift in Anti-Cancer Therapy? Molecules 2018, 23, 1410. [Google Scholar] [CrossRef] [Green Version]
- Zou, T.; Lok, C.-N.; Wan, P.-K.; Zhang, Z.-F.; Fung, S.-K.; Che, C.-M. Anticancer metal-N-heterocyclic carbene complexes of gold, platinum and palladium. Curr. Opin. Chem. Biol. 2018, 43, 30–36. [Google Scholar] [CrossRef]
- Rackham, O.; Nichols, S.J.; Leedman, P.J.; Berners-Price, S.J.; Filipovska, A. A gold(I) phosphine complex selectively induces apoptosis in breast cancer cells: Implications for anticancer therapeutics targeted to mitochondria. Biochem. Pharmacol. 2007, 74, 992–1002. [Google Scholar] [CrossRef]
- Porchia, M.; Pellei, M.; Marinelli, M. New insights in Au-NHCs complexes as anticancer agents. Eur. J. Med. Chem. 2018, 146, 709–746. [Google Scholar] [CrossRef]
- Goetzfried, S.K.; Gallati, C.M.; Cziferszky, M.; Talmazan, R.A.; Wurst, K.; Liedl, K.R.; Podewitz, M.; Gust, R. N-Heterocyclic carbene gold(I) complexes: Mechanism of the ligand scrambling reaction and their oxidation to gold(III) in aqueous solutions. Inorg. Chem. 2020, 59, 15312–15323. [Google Scholar] [CrossRef]
- Onodera, T.; Momose, I.; Kawada, M. Potential anticancer activity of auranofin. Chem. Pharm. Bull. 2019, 67, 186–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radisavljević, S.; Petrović, B. Gold(III) complexes: An overview on their kinetics, interactions with DNA/BSA, cytotoxic activity, and computational calculations. Front. Chem. 2020, 8, 379. [Google Scholar] [CrossRef] [PubMed]
- Quintana, M.; Rodriguez-Rius, A.; Vellé, A.; Vives, S.; Miguel, P.J.S.; Triola, G. Dinuclear silver and gold bisNHC complexes as drug candidates for cancer therapy. Bioorg. Med. Chem. 2022, 67, 116814. [Google Scholar] [CrossRef]
- Meier-Menches, S.M.; Neuditschko, B.; Zappe, K.; Schaier, M.; Gerner, M.; Schmetterer, K.; Del Favero, G.; Bonsignore, R.; Cichna-Markl, M.; Koellensperger, G.; et al. An organometallic gold(I) bis-N-heterocyclic carbene complex with multimodal activity in ovarian cancer cells. Chem. Eur. J. 2020, 26, 15528–15537. [Google Scholar] [CrossRef] [PubMed]
- Ratia, C.; Soengas, R.G.; Soto, S.M. Gold-derived molecules as new antimicrobial agents. Front. Microbiol. 2022, 13, 846959. [Google Scholar] [CrossRef] [PubMed]
- Büssing, R.; Karge, B.; Lippmann, P.; Jones, P.G.; Brönstrup, M.; Ott, I. Gold(I) and gold(III) N-heterocyclic carbene complexes as antibacterial agents and inhibitors of bacterial thioredoxin reductase. ChemMedChem 2021, 16, 3402–3409. [Google Scholar] [CrossRef]
- Glišića, B.Đ.; Djuran, M.I. Gold complexes as antimicrobial agents: An overview of different biological activities in relation to the oxidation state of the gold ion and the ligand structure. Dalton Trans. 2014, 43, 5950–5969. [Google Scholar] [CrossRef]
- Fuchs, B.B.; RajaMuthiah, R.; Souza, A.C.R.; Eatemadpour, S.; Rossoni, R.D.; Santos, D.A.; Junqueira, J.C.; Rice, L.B.; Mylonakis, E. Inhibition of bacterial and fungal pathogens by orphaned drug auronofin. Future Med. Chem. 2016, 8, 117–132. [Google Scholar] [CrossRef] [Green Version]
- Wiederhold, N.P.; Patterson, T.F.; Srinivasan, A.; Chaturvedi, A.K.; Fothergill, A.W.; Wormley, F.L.; Ramasubramanian, A.K.; Lopez-Ribot, J.L. Repurposing auranofin as an antifungal: In vitro activity against a variety of medically important fungi. Virulence 2017, 8, 138–142. [Google Scholar] [CrossRef] [Green Version]
- Frei, A. Metal Complexes, an Untapped Source of Antibiotic Potential? Antibiotics 2020, 9, 90. [Google Scholar] [CrossRef] [Green Version]
- Derbyshire, E.R.; Prudêncio, M.; Mota, M.M.; Clardy, J. Liver-stage malaria parasites vulnerable to diverse chemical scaffolds. Proc. Natl. Acad. Sci. USA 2012, 109, 8511–8516. [Google Scholar] [CrossRef] [Green Version]
- Molter, A.; Rust, J.; Lehmann, C.W.; Deepa, G.; Chiba, P.; Mohr, F. Synthesis, structures and anti-malaria activity of some gold(I) phosphine complexes containing seleno- and thiosemicarbazonato ligands. Dalton Trans. 2011, 10, 9810–9820. [Google Scholar] [CrossRef] [PubMed]
- Khanye, S.D.; Wan, B.; Franzblau, S.G.; Gut, J.; Rosenthal, P.J.; Smith, G.S.; Chibale, K. Synthesis and in vitro antimalarial and antitubercular activity of gold(III) complexes containing thiosemicarbazone ligands. J. Organomet. Chem. 2011, 696, 3392–3396. [Google Scholar] [CrossRef]
- Sannella, A.R.; Casini, A.; Gabbiani, C.; Messori, L. New uses for old drugs. Auranofin, a clinically established antiarthritic metallodrug, exhibits potent antimalarial effects in vitro: Mechanistic and pharmacological implications. FEBS Lett. 2008, 582, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Hemmert, C.; Fabié, A.; Fabre, A.; Benoit-Vical, F.; Gornitzka, H. Synthesis, structures, and antimalarial activities of some silver(I), gold(I) and gold(III) complexes involving N-heterocyclic carbene ligands. Eur. J. Med. Chem. 2013, 60, 64–75. [Google Scholar] [CrossRef]
- Sousa, S.A.; Leitão, J.H.; Silva, R.A.L.; Belo, D.; Santos, I.C.; Guerreiro, J.F.; Martins, M.; Fontinha, D.; Prudêncio, M.; Almeida, M.; et al. On the path to gold: Monoanionic Au bisdithiolate complexes with antimicrobial and antitumor activities. J. Inorg. Biochem. 2020, 202, 110904. [Google Scholar] [CrossRef]
- Fontinha, D.; Sousa, S.A.; Morais, T.S.; Prudêncio, M.; Leitão, J.H.; Le Gal, Y.; Lorcy, D.; Silva, R.A.L.; Velho, M.F.G.; Belo, D.; et al. Gold(III) bis(dithiolene) complexes: From molecular conductors to prospective anticancer, antimicrobial and antiplasmodial agentes. Metallomics 2020, 12, 974–987. [Google Scholar] [CrossRef]
- Marcon, G.; Messori, L.; Orioli, P.; Cinellu, M.A.; Minghetti, G. Reactions of gold(III) complexes with serum albumin. Eur. J. Biochem. 2003, 270, 4655–4661. [Google Scholar] [CrossRef] [Green Version]
- Topală, T.; Bodoki, A.; Oprean, L.; Oprean, R. Bovine Serum Albumin Interactions with Metal Complexes. Clujul Med. 2014, 87, 215–219. [Google Scholar] [CrossRef] [Green Version]
- Eid, S.; Fourmigué, M.; Roisnel, T.; Lorcy, D. Influence of the thiazole backbone on the structural, redox, and optical properties of dithiolene and diselenolene complexes. Inorg. Chem. 2007, 46, 10647–10654. [Google Scholar] [CrossRef]
- Filatre-Furcate, A.; Barrière, F.; Roisnel, T.; Jeannin, O.; Lorcy, D. Conformational behavior, redox and spectroscopic properties of gold dithiolene complexes: [Au(iPr-thiazYdt)2]-1 (Y = O, S, Se]. Inorg. Chim. Acta 2018, 469, 255–263. [Google Scholar] [CrossRef]
- Busch, W.; Duis, K.; Fenske, M.; Maack, G.; Legler, J.; Padilla, S.; Strähle, U.; Witters, H.; Scholz, S. The zebrafish embryo model in toxicology and teratology. Reprod. Toxicol. 2011, 31, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Chaves, R.S.; Guerreiro, C.S.; Cardoso, V.V.; Benoliel, M.J.; Santos, M.M. Toxicological assessment of seven unregulated drinking water disinfection by-products (DBPs) using the zebrafish embryo bioassay. Sci. Total Environ. 2020, 742, 140522. [Google Scholar] [CrossRef] [PubMed]
- Hanigan, D.; Truong, L.; Simonich, M.; Tanguay, R.; Westerhoff, P. Zebrafish embryo toxicity of 15 chlorinated, brominated, and iodinated disinfection by-products. J. Environ. Sci. 2017, 58, 302–310. [Google Scholar] [CrossRef]
- Ribeiro, S.; Torres, T.; Martins, R.; Santos, M.M. Toxicity screening of Diclofenac, Propranolol, Sertraline and Simvastatin using Danio rerio and Paracentrotus lividus embryo bioassays. Ecotox. Environ. Saf. 2015, 114, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.-P.; Feng, F.; Zhang, X.-Q.; Liu, X.-X.; Wang, Y.-B.; She, J.-X.; He, Z.-H.; He, M.-F. Toxicity Assessment of 7 Anticancer Compounds in Zebrafish. Int. J. Toxicol. 2014, 33, 98–105. [Google Scholar] [CrossRef]
- Chirullo, B.; Sgarbanti, R.; Limongi, D.; Shytaj, I.L.; Alvarez, D.; Das, B.; Boe, A.; DaFonseca, S.; Chomont, N.; Liotta, L.; et al. A candidate anti-HIV reservoir compound, auranofin, exerts a selective ‘anti-memory’ effect by exploiting the baseline oxidative status of lymphocytes. Cell Death Dis. 2013, 4, e944. [Google Scholar] [CrossRef] [Green Version]
- Rigobello, M.P.; Messori, L.; Marcon, G.; Cinellu, M.A.; Bragadin, M.; Folda, A.; Scutari, G.; Bindoli, A. Gold complexes inhibit mitochondrial thioredoxin reductase: Consequences on mitochondrial functions. J. Inorg. Biochem. 2004, 98, 1634–1641. [Google Scholar] [CrossRef]
- Pratesi, A.; Gabbiani, C.; Michelucci, E.; Ginanneschi, M.; Papini, A.M.; Rubbiani, R.; Ott, I.; Messori, L. Insights on the mechanism of thioredoxin reductase inhibition by gold N-heterocyclic carbene compounds using the synthetic linear selenocysteine containing C-terminal peptide hTrxR(488-499): An ESI-MS investigation. J. Inorg. Biochem. 2014, 136, 161–169. [Google Scholar] [CrossRef]
- Bian, M.; Fan, R.; Zhao, S.; Liu, W. Targeting the thioredoxin system as a strategy for cancer therapy. J. Med. Chem. 2019, 62, 7309–7321. [Google Scholar] [CrossRef]
- Becker, K.; Gromer, S.; Schirmer, R.H.; Müller, S. Thioredoxin reductase as a pathophysiological factor and drug target. Eur. J. Biochem. 2000, 267, 6118–6125. [Google Scholar] [CrossRef] [PubMed]
- Ashall, F. Cancer cells and parasites: Two of a kind. Trends Biochem. Sci. 1986, 11, 518–520. [Google Scholar] [CrossRef]
- Duesberg, P.; Madrioli, D.; McCormack, A.; Nicholson, J.M. Is carcinogenesis a form of speciation? Cell Cycle 2011, 10, 2100–2114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckmann, S.; Grevelding, C.G. Imatinib makes a fatal impact on morphology, pairing stability, and survival of adult Schistosoma mansoni in vitro. Int. J. Parasitol. 2010, 40, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Harbut, M.B.; Vilchèze, C.; Luo, X.; Hensler, M.E.; Guo, H.; Yang, B.; Chatterjee, A.K.; Nizet, V.; Jacobs, W.R.; Schultz, P.G.; et al. Auranofin exerts broad-spectrum bactericidal activities by targeting thiol-redox homeostasis. Proc. Natl. Acad. Sci. USA 2015, 112, 4453–4458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morais, T.S.; Santos, F.C.; Corte-Real, L.; Garcia, M.H. Exploring the effect of the ligand design on the interactions between [Ru(η5-C5H5)(PPh3)(N,O)][CF3SO3] complexes and human serum albumin. J. Inorg. Biochem. 2013, 129, 94–101. [Google Scholar] [CrossRef]
- García-Moreno, E.; Tomás, A.; Atrián-Blasco, E.; Gascon, S.; Romanos, E.; Rodriguez-Yoldi, M.J.; Cerrada, E.; Laguna, M. In vitro and in vivo evaluation of organometallic gold(I) derivatives as anticancer agents. Dalton Trans. 2016, 45, 2462–2475. [Google Scholar] [CrossRef] [Green Version]
- Gouvea, L.R.; Garcia, L.S.; Lachter, D.R.; Nunes, P.R.; de Castro Pereira, F.; Silveira-Lacerda, E.P.; Louro, S.R.W.; Jorge, P.; Barbeira, S.; Teixeira, L.R. Atypical fluoroquinolone gold(III) chelates as potential anticancer agents: Relevance of DNA and protein interactions for their mechanism of action. Eur. J. Med. Chem. 2012, 55, 67–73. [Google Scholar] [CrossRef]
- Tunes, L.G.; Morato, R.E.; Garcia, A.; Schmitz, V.; Steindel, M.; Corrêa-Junior, J.D.; Dos Santos, H.F.; Frézard, F.; de Almeida, M.V.; Silva, H.; et al. Preclinical gold complexes as oral drug candidates to treat leishmaniasis are potent trypanothione reductase inhibitors. ACS Infect. Dis. 2020, 6, 1121–1139. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Ding, F.; Diao, J.; Yang, X.-L.; Sun, Y. Structural analysis and binding domain of albumin complexes with natural dietary supplement humic acid. J. Lumin. 2011, 131, 2244–2251. [Google Scholar] [CrossRef]
- Le Gal, Y.; Rajkumar, M.; Vacher, A.; Dorcet, V.; Roisnel, T.; Fourmigué, M.; Barrière, F.; Guizouarn, T.; Lorcy, D. A sulfur-rich π-electron acceptor derived from 5,5’-bithiazolidinylidene: Charge-transfer complex vs charge-transfer salt. CrystEngComm 2016, 18, 3925–3933. [Google Scholar] [CrossRef]
- Le Gal, Y.; Roisnel, T.; Auban-Senzier, P.; Bellec, M.; Íñiguez, J.; Canadell, E.; Lorcy, D. Stable metallic state of a neutral radical single-component conductor at ambient pressure. J. Am. Chem. Soc. 2018, 140, 6998–7004. [Google Scholar] [CrossRef] [PubMed]
- Tenn, N.; Bellec, N.; Jeannin, O.; Piekara-Sady, L.; Auban-Senzier, P.; Íñiguez, J.; Canadell, E.; Lorcy, D. A single-component molecular metal based on a thiazole dithiolate gold complex. J. Am. Chem. Soc. 2009, 131, 16961–16967. [Google Scholar] [CrossRef] [PubMed]
- Yzambart, G.; Bellec, N.; Nasser, G.; Jeannin, O.; Roisnel, T.; Fourmigué, M.; Auban-Senzier, P.; Íñiguez, J.; Canadell, R.; Lorcy, D. Anisotropic chemical pressure effects in single-component molecular metals based on radical dithiolene and diselenolene gold complexes. J. Am. Chem. Soc. 2012, 134, 17138–17148. [Google Scholar] [CrossRef] [PubMed]
- Filatre-Furcate, A.; Roisnel, T.; Fourmigué, M.; Jeannin, O.; Bellec, N.; Auban-Senzier, P.; Lorcy, D. Subtle steric differences impact the structural and conducting properties of radical gold bis(dithiolene) complexes. Chem. Eur. J. 2017, 23, 16004–16013. [Google Scholar] [CrossRef]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Cryst. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar]
- Nakayama, G.R.; Caton, M.C.; Nova, M.P.; Parandoosh, Z. Assessment of the Alamar Blue assay for cellular growth and viability in vitro. J. Immunol. Methods 1997, 204, 205–208. [Google Scholar] [CrossRef]
- Ploemen, I.H.J.; Prudêncio, M.; Douradinha, B.G.; Ramesar, J.; Fonager, J.; van Gemert, G.-J.; Luty, A.J.F.; Hermsen, C.C.; Sauerwein, R.W.; Baptista, F.G.; et al. Visualisation and quantitative analysis of the rodent malaria liver stage by real time imaging. PLoS ONE 2009, 4, e7881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duthie, E.S.; Lorenz, L.L. Staphylococcal coagulase: Mode of action and antigenicity. J. Gen. Microbiol. 1952, 6, 95–107. [Google Scholar] [CrossRef] [Green Version]
- Minogue, T.D.; Daligault, H.A.; Davenport, K.W.; Bishop-Lilly, K.A.; Broomall, S.M.; Bruce, D.C.; Chain, P.S.; Chertkov, O.; Coyne, S.R.; Freitas, T.; et al. Complete genome assembly of Escherichia coli ATCC 25922, a serotype O6 reference strain. Genome Announc. 2014, 2, e00969-14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borst, A.; Raimer, M.T.; Warnock, D.W.; Morrison, C.J.; Arthington-Skaggs, B.A. Rapid acquisition of stable azole resistance by Candida glabrata isolates obtained before the clinical introduction of fluconazole. Antimicrob. Agents Chemother. 2005, 49, 783–787. [Google Scholar] [CrossRef] [Green Version]
- Gillum, A.M.; Tsay, E.Y.; Kirsch, D.R. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol. Gen. Genet. 1984, 198, 179–182. [Google Scholar] [CrossRef]
- Weinstein, M.P.; Patel, J.B.; Burnham, C.-A.; Campeau, S.; Conville, P.S.; Doern, C.; Eliopoulos, G.M.; Galas, M.F.; Humphries, R.M.; Jenkins, S.G.; et al. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; CLSI Supplement M07; Wayne, P.A., Ed.; Clinical and Laboratory Standards Institute: Annapolis Junction, MD, USA, 2018. [Google Scholar]
- Arendrup, M.C.; Cuenca-Estrella, M.; Lass-Flörl, C.; Hope, W. EUCAST technical note on the EUCAST definitive document EDef 7.2: Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef7.2 (EUCAST-AFST). Clin. Microbiol. Infect. 2012, 18, E246–E247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, R.J.W.; Pearson, J. Susceptibility testing: Accurate and reproducible minimum inhibitory concentration (MIC) and non-inhibitory concentration (NIC) values. J. Appl. Microbiol. 2000, 88, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Bártolo, I.; Santos, B.S.; Fontinha, D.; Machado, M.; Francisco, D.; Sepodes, B.; Rocha, J.; Mota-Filipe, H.; Pinto, R.; Figueira, M.E.; et al. Spiro-beta-lactam BSS-730A displays potent activity against HIV and Plasmodium. ACS Infect. Dis. 2021, 7, 421–434. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Holmgren, A.; Björnstedt, M. Thioredoxin and thioredoxin reductase. Methods Enzymol. 1995, 252, 199–208. [Google Scholar]
- Beaven, G.H.; Chen, S.-H.; D’albis, A.; Gratzer, W.B. A spectroscopic study of the haemin—Human-serum-albumin system. Eur. J. Biochem. 1974, 41, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, A.; Prieto, M. Ribonuclease T1 and alcohol dehydrogenase fluorescence quenching by acrylamide: A laboratory experiment for undergraduate students. J. Chem. Educ. 1993, 70, 425–428. [Google Scholar] [CrossRef]
- Kubista, M.; Sjöback, R.; Eriksson, S.; Albinsson, B. Experimental correction for the inner-filter effect in fluorescence spectra. Analyst 1994, 119, 417–419. [Google Scholar] [CrossRef]












| Complexes | E−2/−1 | E1/2−1/0 | Epa2/Epc20/+1 |
|---|---|---|---|
| [AuSMe(=S)]−1 | −0.85 | 0.51/0.44 * | 0.73/0.56 * |
| [AuSEt(=S)]−1 | −0.90 | 0.55/0.49 * | 0.71/0.61 * |
| [AuSPr(=S)]−1 | −0.85 ** | 0.56/0.53 * | 0.73/0.64 |
| [AuSBu(=S)]−1 | −0.88 ** | 0.55/0.50 | 0.75/0.66 * |
| [AuSEt(=O)]−1 | −1.05 ** | 0.42/0.36 | 0.89/0.83 |
| [AuSEt(=C(CN)2)]−1 | − | 0.71/0.66 | 1.13/1.06 |
| [AuSeEt(=Se)]−1 | −0.84 ** | 0.43/− ** | − |
| [AuSEt(=Se)]−1 | −0.80 ** | 0.40/− ** | − |
| [AuSPr(=Se)]−1 | −0.84 ** | 0.45/− ** | − |
| [AuSeEt(=S)]−1 | −0.89 ** | 0.52/− ** | − |
| [AuSePr(=S)]−1 | −0.90 | 0.54/− ** | 0.95/− |
| [AuSeiPr(=S)]−1 | −0.94 | 0.62/0.57 * | 0.75/− |
 | IC50 (µM) | ||
|---|---|---|---|
| A2780, 24 h | A2780, 48 h | OVCAR8, 48 h | |
| [N][AuSMe(=S)] | 1.63 ± 0.40 | 0.11 ± 0.0 | 2.44 ± 0.78 |
| [N][AuSEt(=S)] | 4.23 ± 0.80 * | 1.30 ± 0.32 * | 3.76 ± 0.71 |
| [P][AuSEt(=S)] | 0.58 ± 0.20 | 0.18 ± 0.0 | 0.85 ± 0.19 |
| [P][AuSEt(=O)] | 0.86 ± 0.11 | 0.31 ± 0.10 | 1.16 ± 0.21 |
| [P][AuSEt(=Se)] | 0.71 ± 0.12 | 0.22 ± 0.11 | 0.97 ± 0.18 |
| [N][AuSEt(=C(CN)2)] | 1.32 ± 0.20 | 0.17 ± 0.0 | 1.48 ± 0.41 |
| [N][AuSeEt(=S)] | 19.2 ± 2.01 | 4.32 ± 1.17 | 29.4 ± 4.20 |
| [N][AuSeEt(=Se)] | 26.9 ± 3.02 | 4.84 ± 1.60 | 13.9 ± 1.61 |
| [P][AuSeEt(=Se)] | 3.96 ± 2.21 | 0.64 ± 0.19 | 2.42 ± 1.52 |
| [N][AuSPr(=S)] | 2.93 ± 0.81 | 2.28 ± 0.69 | 2.28 ± 0.19 |
| [P][AuSPr(=S)] | 4.60 ± 1.15 * | 0.52 ± 0.23 * | 1.04 ± 3.91 |
| [P][AuSPr(=Se)] | 0.62 ± 0.19 | 0.55 ± 0.41 | 0.80 ± 0.18 |
| [P][AuSePr(=S)] | 0.91 ± 0.40 | 0.29 ± 0.10 | 1.57 ± 0.43 |
| [N][AuSeiPr(=S)] | 30.3 ± 3.39 | 8.27 ± 1.12 | 8.76 ± 0.69 |
| [P][AuSeiPr(=S)] | 3.74 ± 1.22 | 0.68 ± 0.18 | 3.86 ± 0.91 |
| [P][AuSBu(=S)] | 1.38 ± 0.28 | 0.95 ± 0.05 | 1.41 ± 0.56 |
| Auranofin | 2.01 ± 0.71 | 0.43 ± 0.27 * | 1.04 ± 0.41 |
| Cisplatin | 21.1 ± 5.0 | 3.60 ± 1.25 * | 10.1 ± 2.3 |
 | IC50 (nM) | |
|---|---|---|
| P. berghei | PfNF54 | |
| [P][AuSEt(=Se)] | 415.67 ± 122.73 | 1002.30 ± 391.31 |
| [N][AuSMe(=S)] | 428.73 ± 39.62 | ND |
| [P][AuSBu(=S)] | 469.57 ± 114.63 | ND |
| [P][AuSEt(=S)] | 474.00 ± 109.77 | 1062.25 ± 392.80 |
| [P][AuSeEt(=Se)] | 517.83 ± 245.87 | ND |
| [P][AuSePr(=S)] | 543.60 ± 19.37 | ND |
| [P][AuSPr(=Se)] | 643.85 ± 1.34 | ND |
| [P][AuSeiPr(=S)] | 748.45 ± 70.22 | ND |
 | MIC (µg/mL) | |||
|---|---|---|---|---|
| S. aureus Newman | E. coli ATCC25922 | C. albicans SC5134 | C. glabrata CBS138 | |
| [N][AuSMe(=S)] | 4.4 ± 0.2 | >250 | >62.5 *** | 2.9 ± 0.9 |
| [N][AuSEt(=S)] * | 12.1 ± 3.9 | >125 | 19.9 ± 2.4 | 9.7 ± 2.7 |
| [P][AuSEt(=S)] | >125 | >125 | >62.5 | >62.5 |
| [P][AuSEt(=O)] | >125 | >125 | >62.5 | >62.5 |
| [P][AuSEt(=Se)] | >125 | >125 | >62.5 | >62.5 |
| [N][AuSEt(=C(CN)2)] | >125 | >125 | >62.5 | >62.5 |
| [N][AuSPr(=S)] | 1.1 ± 0.2 | >250 | 3.1 ± 0.5 | 1.6 ± 0.8 |
| [P][AuSPr(=S)] ** | >125 | >125 | >125 | >125 |
| [P][AuSPr(=Se) | >125 | >125 | 62.5 | >62.5 |
| [P][AuSBu(=S)] | >125 | >125 | >62.5 | >62.5 |
| [N][AuSeEt(=S)] | 5.5 ± 0.3 | >125 | >62.5 | 1.6 ± 0.8 |
| [N][AuSeiPr(=S)] | 1.5 ± 0.1 | >250 | >125 | 2.1 ± 0.1 |
| [P][AuSeiPr(=S)] | >62.5 | >62.5 | >31.25 | >31.25 |
| [P][AuSePr(=S)] | >250 | >250 | >125 | >125 |
| [N][AuSeEt(=Se)] | >62.5 | >62.5 | >31.25 | 1.8 ± 0.5 |
| [P][AuSeEt(=Se)] | >125 | >125 | >62.5 | >62.5 |
| Auranofin | 0.173 ± 0.004 | 35.5 ± 0.6 | 7.9 ± 0.5 | 15.2 ± 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Gal, Y.; Filatre-Furcate, A.; Lorcy, D.; Jeannin, O.; Roisnel, T.; Dorcet, V.; Fontinha, D.; Francisco, D.; Prudncio, M.; Martins, M.; et al. Broad Spectrum Functional Activity of Structurally Related Monoanionic Au(III) Bis(Dithiolene) Complexes. Int. J. Mol. Sci. 2022, 23, 7146. https://doi.org/10.3390/ijms23137146
Le Gal Y, Filatre-Furcate A, Lorcy D, Jeannin O, Roisnel T, Dorcet V, Fontinha D, Francisco D, Prudncio M, Martins M, et al. Broad Spectrum Functional Activity of Structurally Related Monoanionic Au(III) Bis(Dithiolene) Complexes. International Journal of Molecular Sciences. 2022; 23(13):7146. https://doi.org/10.3390/ijms23137146
Chicago/Turabian StyleLe Gal, Yann, Agathe Filatre-Furcate, Dominique Lorcy, Olivier Jeannin, Thierry Roisnel, Vincent Dorcet, Diana Fontinha, Denise Francisco, Miguel Prudncio, Marta Martins, and et al. 2022. "Broad Spectrum Functional Activity of Structurally Related Monoanionic Au(III) Bis(Dithiolene) Complexes" International Journal of Molecular Sciences 23, no. 13: 7146. https://doi.org/10.3390/ijms23137146