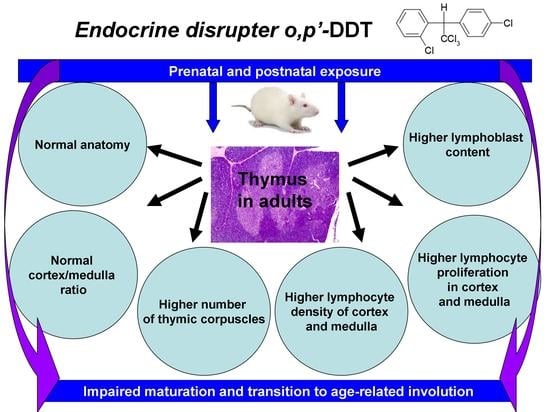
Developmental Exposure to Endocrine Disrupter DDT Interferes with Age-Related Involution of Thymus
Abstract
:1. Introduction
2. Results
2.1. Thymus Morphology
2.2. Proliferation of Thymic Lymphocytes
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Thymus Morphology
4.4. Immunohistochemistry
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anderson, G.; Moore, N.C.; Owen, J.J.; Jenkinson, E.J. Cellular interactions in thymocyte development. Annu. Rev. Immunol. 1996, 14, 73–99. [Google Scholar] [CrossRef] [PubMed]
- Thapa, P.; Farber, D.L. The role of the thymus in the immune response. Thorac. Surg. Clin. 2019, 29, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Calder, A.E.; Hince, M.N.; Dudakov, J.A.; Chidgey, A.P.; Boyd, R.L. Thymic involution: Where endocrinology meets immunology. Neuroimmunomodulation 2011, 18, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Goronzy, J.J.; Weyand, C.M. Aging, autoimmunity and arthritis: T-cell senescence and contraction of T-cell repertoire diversity—catalysts of autoimmunity and chronic inflammation. Arthritis Res. Ther. 2003, 5, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Sheu, T.T.; Chiang, B.L.; Yen, J.H.; Lin, W.C. Premature CD4. T cell aging and its contribution to lymphopenia-induced proliferation of memory cells in autoimmune-prone non-obese diabetic mice. PLoS ONE 2014, 9, e89379. [Google Scholar] [CrossRef] [Green Version]
- Asher, M.I.; Montefort, S.; Bjorksten, B.; Lai, C.K.; Strachan, D.P.; Weiland, S.K.; Williams, H. ISAAC Phase Three Study Group. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet 2006, 368, 733–743. [Google Scholar] [CrossRef]
- Somova, L.M.; Kotsyurbiy, E.A.; Drobot, E.I.; Lyapun, I.N.; Shchelkanov, M.Y. Clinical and morphological manifestations of immune system dysfunction in new coronavirus infection (COVID-19). Clin. Exp. Morphol. 2021, 10, 11–20. [Google Scholar] [CrossRef]
- Nakamura, K.; Kariyazono, H. Influence of endocrine-disrupting chemicals on the immune system. J. Health Sci. 2010, 56, 361–373. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.-H.; Yang, S.-N.; Kuo, P.-L.; Hung, C.-H. Immunomodulatory effects of environmental endocrine disrupting chemicals. Kaohsiung J. Med. Sci. 2012, 28 (Suppl. 7), S37–S42. [Google Scholar] [CrossRef] [Green Version]
- Chittrakul, J.; Sapbamrer, R.; Sirikul, W. Pesticide Exposure and Risk of Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Toxics 2022, 10, 207. [Google Scholar] [CrossRef]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2, The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [CrossRef]
- Jaakkola, J.J.; Knight, T.L. The role of exposure to phthalates from polyvinyl chloride products in the development of asthma and allergies: A systematic review and meta-analysis. Environ. Health Perspect. 2008, 116, 845–853. [Google Scholar] [CrossRef]
- Larsson, M.; Hagerhed-Engman, L.; Kolarik, B.; James, P.; Lundin, F.; Janson, S.; Sundell, J.; Bornehag, S.G. PVCeas flooring materialeand its association with incident asthma in a Swedish child cohort study. Indoor Air 2010, 20, 494–501. [Google Scholar] [CrossRef]
- Makene, V.; Pool, E. The Effects of Endocrine Disrupting Chemicals on Biomarkers of Inflammation Produced by Lipopolysaccharide Stimulated RAW264.7 Macrophages. Int. J. Environ. Res. Public Health 2019, 16, 2914. [Google Scholar] [CrossRef] [Green Version]
- De Mello-Coelho, V.; Gagnerault, M.C.; Souberbielle, J.C.; Strasburger, C.J.; Savino, W.; Dardenne, M.; Postel-Vinay, M.C. Growth hormone and its receptor are expressed in human thymic cells. Endocrinology 1998, 139, 3837–3842. [Google Scholar] [CrossRef]
- Savino, W.; Dardenne, M. Neuroendocrine Control of Thymus Physiology. Endocr. Rev. 2000, 21, 412–443. [Google Scholar] [CrossRef]
- Olsen, N.J.; Viselli, S.M.; Fan, J.; Kovacs, W.J. Androgens accelerate thymocyte apoptosis. Endocrinology 1998, 139, 748–752. [Google Scholar] [CrossRef]
- Rijhsinghani, A.G.; Thompson, K.; Bhatia, S.K.; Waldschmidt, T.J. Estrogen blocks early T cell development in the thymus. Am. J. Reprod. Immunol. 1996, 36, 269–277. [Google Scholar] [CrossRef]
- Bräuner, E.V.; Uldbjerg, C.S.; Lim, Y.H.; Gregersen, L.S.; Krause, M.; Frederiksen, H.; Andersson, A.M. Presence of parabens, phenols and phthalates in paired maternal serum, urine and amniotic fluid. Environ. Int. 2022, 158, 106987. [Google Scholar] [CrossRef]
- Mestres, J.; Pérez-Albaladejo, E.; Porte, C.; Postigo, C. High-throughput analysis of the steroid profile in placental cell cultures to evaluate endocrine disrupting effects of contaminant exposure. J. Chromatogr. A 2022, 1667, 462886. [Google Scholar] [CrossRef]
- Street, M.E.; Angelini, S.; Bernasconi, S.; Burgio, E.; Cassio, A.; Catellani, C.; Cirillo, F.; Deodati, A.; Fabbrizi, E.; Fanos, V.; et al. Current knowledge on endocrine disrupting chemicals (EDCs) from animal biology to humans, from pregnancy to adulthood: Highlights from a national Italian meeting. Int. J. Mol. Sci. 2018, 19, 1647. [Google Scholar] [CrossRef] [Green Version]
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat. Rev. Endocrinol. 2020, 18, 45–57. [Google Scholar] [CrossRef] [Green Version]
- Yaglova, N.V.; Tsomartova, E.S.; Nazimova, S.V.; Obernikhin, S.S.; Mukhamedova, S.G.; Pashina, N.R.; Musaeva, D.O. Morphological Changes in the Thymus of Newborn Rats Exposed to Endocrine Disruptor Dichlorodiphenyltrichloroethane (DDT) during the Prenatal Period. Bull. Exp. Biol. Med. 2019, 167, 297–299. [Google Scholar] [CrossRef]
- World Health Organization. Pesticide Residues in Food—2018. Toxicological Evaluations; World Health Organization and Food and Agriculture Organization of the United Nations—WHO: Geneva, Switzerland, 2019; 780p. [Google Scholar]
- Disruptors, E. From Scientific Evidence to Human Health Protection; Policy Department for Citizens’ Rights and Constitutional Affairs, Directorate General for Internal Policies of the Union: Strasbourg, France, 2019; 132p. [Google Scholar]
- Roy, J.R.; Chakraborty, S.; Chakraborty, T.R. Estrogen-like endocrine disrupting chemicals affecting puberty in humans—A review. Med. Sci. Monit. 2009, 15, 137–145. [Google Scholar]
- Ozen, S.; Darcan, S. Effects of Environmental Endocrine Disruptors on Pubertal Development. J. Clin. Res. Ped. Endocrinol. 2011, 3, 1–6. [Google Scholar] [CrossRef]
- Gruver, A.L.; Sempowski, G.D. Cytokines, leptin, and stress-induced thymic atrophy. J. Leukoc. Biol. 2008, 84, 915–923. [Google Scholar] [CrossRef]
- Simon, A.K.; Hollander, G.A.; McMichael, A. Evolution of the immune system in humans from infancy to old age. Proc. Biol. Sci. 2015, 282, 20143085. [Google Scholar] [CrossRef]
- Sutherland, J.S.; Goldberg, G.L.; Hammett, M.V.; Uldrich, A.P.; Berzins, S.P.; Heng, T.S.; Blazar, B.R.; Millar, J.L.; Malin, M.A.; Chidgey, A.P.; et al. Activation of thymic regeneration in mice and humans following androgen blockade. J. Immunol. 2005, 175, 2741–2753. [Google Scholar] [CrossRef]
- Goldberg, G.L.; King, C.G.; Nejat, R.A.; Suh, D.Y.; Smith, O.M.; Bretz, J.C.; Samstein, R.M.; Dudakov, J.A.; Chidgey, A.P.; Chen-Kiang, S.; et al. Luteinizing hormone-releasing hormone enhances T cell recovery following allogeneic bone marrow transplantation. J. Immunol. 2009, 182, 5846–5854. [Google Scholar] [CrossRef] [Green Version]
- Kelce, W.R.; Stone, C.R.; Laws, S.C.; Gray, L.E.; Kemppainen, J.A.; Wilson, E.M. Persistent DDT metabolite p,p’-DDE is a potent androgen receptor antagonist. Nature 1995, 375, 581–585. [Google Scholar] [CrossRef]
- Tsomartova, D.A.; Yaglova, N.V.; Yaglov, V.V. Changes in canonical-catenin/Wnt signaling activation in the adrenal cortex of rats exposed to endocrine disruptor dichlorodiphenyltrichloroethane (DDT) during prenatal and postnatal ontogeny. Bull. Exp. Biol. Med. 2018, 164, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Yaglova, N.V.; Obernikhin, S.S.; Tsomartova, D.A.; Nazimova, S.V.; Yaglov, V.V.; Tsomartova, E.S.; Chereshneva, E.V.; Ivanova, M.Y.; Lomanovskaya, T.A. Impaired Morphogenesis and Function of Rat Adrenal Zona Glomerulosa by Developmental Low-Dose Exposure to DDT Is Associated with Altered Oct4 Expression. Int. J. Mol. Sci. 2021, 22, 6324. [Google Scholar] [CrossRef] [PubMed]
- Yaglova, N.V.; Obernikhin, S.S.; Yaglov, V.V.; Nazimova, S.V.; Timokhina, E.P.; Tsomartova, D.A. Low-Dose Exposure to Endocrine Disrupter Dichlorodiphenyltrichloroethane (DDT) Affects Transcriptional Regulation of Adrenal Zona Reticularis in Male Rats. Bull. Exp. Biol. Med. 2021, 170, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Gruver, A.; Hudson, L.; Sempowski, G.D. Immunosenescence of ageing. J. Pathol. 2007, 211, 144–156. [Google Scholar] [CrossRef]
- Takahama, Y. Journey through the thymus: Stromal guides for T-cell development and selection. Nat. Rev. Immunol. 2006, 6, 127. [Google Scholar] [CrossRef]
- Kondo, K.; Ohigashi, I.; Takahama, Y. Thymus machinery for T-cell selection. Int. Immunol. 2019, 31, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Bodey, B.; Bodey, B., Jr.; Siegel, S.E.; Kaiser, H.E. Novel insights into the function of the thymic Hassall’s bodies. In Vivo 2000, 14, 407–418. [Google Scholar]
- Wang, J.; Sekai, M.; Matsui, T.; Fujii, Y.; Matsumoto, M.; Takeuchi, O.; Minato, N.; Hamazaki, Y. Hassall’s corpuscles with cellular-senescence features maintain IFNα production through neutrophils and pDC activation in the thymus. Int. Immunol. 2019, 31, 127–139. [Google Scholar] [CrossRef] [Green Version]
- Laan, M.; Salumets, A.; Klein, A.; Reintamm, K.; Bichele, R.; Peterson, H.; Peterson, P. Post-Aire Medullary Thymic Epithelial Cells and Hassall’s Corpuscles as Inducers of Tonic Pro-Inflammatory Microenvironment. Front. Immunol. 2021, 12, 635569. [Google Scholar] [CrossRef]
- Bodey, B.; Kaiser, H.E. Development of Hassall’s bodies of the thymus in humans and other vertebrates (especially mammals) under physiological and pathological conditions: Immunocytochemical, electron microscopic and in vitro observations. In Vivo 1997, 11, 61–85. [Google Scholar]
- Yaglova, N.V.; Tsomartova, E.S.; Obernikhin, S.S.; Ivanova, M.Y.; Chereshneva, E.V.; Muhamedova, S.G.; Lomanovskaya, T.A.; Yaglov, V.V. Developmental exposure to low doses of dichlorodiphenyltrichloroethane impairs proliferative response of thymic lymphocytes to Concanavalin A in rats. Heliyon 2020, 6, e03608. [Google Scholar] [CrossRef]
- Holladay, S.; Smialowicz, R. Development of the murine and human immune system: Differential effects of immunotoxicants depend on time of exposure. Environ. Health Perspect. 2000, 108, 463–473. [Google Scholar] [CrossRef] [Green Version]
- Haynes, L.; Eaton, S.M.; Burns, E.M.; Randall, T.D.; Swain, S.L. CD4 T cell memory derived from young naive cells functions well into old age, but memory generated from aged naive cells functions poorly. Proc. Natl. Acad. Sci. USA 2003, 100, 15053–15058. [Google Scholar] [CrossRef] [Green Version]
- Sansoni, P.; Vescovini, R.; Fagnoni, F.; Biasini, C.; Zanni, F.; Zanlari, L.; Telera, A.; Lucchini, G.; Passeri, G.; Monti, D.; et al. The immune system in extreme longevity. Exp. Gerontol. 2008, 43, 61–65. [Google Scholar] [CrossRef]
- Damgaard, I.N.; Skakkebaek, N.E.; Toppari, J.; Virtanen, H.E.; Shen, H.; Schramm, K.W.; Petersen, J.H.; Jensen, T.K.; Main, K.M. Persistent pesticides in human breast milk and cryptorchidism. Environ. Health Perspect. 2006, 114, 1133–1138. [Google Scholar] [CrossRef] [Green Version]
- Vested, A.; Ramlau-Hansen, C.H.; Olsen, S.F.; Bonde, J.P.; Støvring, H.; Kristensen, S.L.; Halldorsson, T.I.; Rantakokko, P.; Kiviranta, H.; Ernst, E.H.; et al. In Utero Exposure to Persistent Organochlorine Pollutants and Reproductive Health in the Human Male. Reproduction 2014, 148, 635–646. [Google Scholar] [CrossRef] [Green Version]
- Yaglova, N.V.; Obernikhin, S.S.; Tsomartova, D.A.; Nazimova, S.V.; Yaglov, V.V. Expression of Transcription Factor PRH/Hhex in Adrenal Chromaffin Cells in the Postnatal Development and Its Role in the Regulation of Proliferative Processes. Bull. Exp. Biol. Med. 2018, 165, 508–511. [Google Scholar] [CrossRef]
- Yaglova, N.V.; Obernikhin, S.S.; Nazimova, S.V.; Yaglov, V.V. Developmental exposure to endocrine disrupter dichlorodiphenyltrichloroethane alters transcriptional regulation of postnatal morphogenesis of adrenal zona fasciculata. Saudi J. Biol. Sci. 2020, 27, 3655–3659. [Google Scholar] [CrossRef]
- Yamazaki, H.; Takano, R.; Shimizu, M.; Murayama, N.; Kitajima, M.; Shono, M. Human blood concentrations of dichlorodiphenyltrichloroethane (DDT) extrapolated from metabolism in rats and humans and physiologically based pharmacokinetic modeling. J. Health Sci. 2010, 56, 566–575. [Google Scholar] [CrossRef] [Green Version]
- Yaglova, N.V.; Obernikhin, S.S. Morphofunctional changes in thymic offspring of mice in the period of puberty and in adults after a single immunostimulatory effects of the parent organism in the early stages of pregnancy. Immunologiya 2013, 34, 15–19. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaglova, N.V.; Obernikhin, S.S.; Tsomartova, E.S.; Yaglov, V.V.; Nazimova, S.V.; Tsomartova, D.A.; Timokhina, E.P.; Chereshneva, E.V.; Ivanova, M.Y.; Payushina, O.V. Developmental Exposure to Endocrine Disrupter DDT Interferes with Age-Related Involution of Thymus. Int. J. Mol. Sci. 2022, 23, 6678. https://doi.org/10.3390/ijms23126678
Yaglova NV, Obernikhin SS, Tsomartova ES, Yaglov VV, Nazimova SV, Tsomartova DA, Timokhina EP, Chereshneva EV, Ivanova MY, Payushina OV. Developmental Exposure to Endocrine Disrupter DDT Interferes with Age-Related Involution of Thymus. International Journal of Molecular Sciences. 2022; 23(12):6678. https://doi.org/10.3390/ijms23126678
Chicago/Turabian StyleYaglova, Nataliya V., Sergey S. Obernikhin, Elina S. Tsomartova, Valentin V. Yaglov, Svetlana V. Nazimova, Dibakhan A. Tsomartova, Ekaterina P. Timokhina, Elizaveta V. Chereshneva, Marina Y. Ivanova, and Olga V. Payushina. 2022. "Developmental Exposure to Endocrine Disrupter DDT Interferes with Age-Related Involution of Thymus" International Journal of Molecular Sciences 23, no. 12: 6678. https://doi.org/10.3390/ijms23126678