Rigid Schiff Base Complex Supermolecular Aggregates as a High-Performance pH Probe: Study on the Enhancement of the Aggregation-Caused Quenching (ACQ) Effect via the Substitution of Halogen Atoms
Abstract
:1. Introduction
2. Results
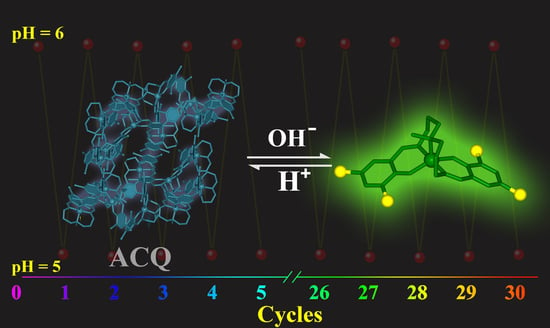
2.1. Photophysical Properties
2.2. Single-Crystal X-ray Diffraction Analyses
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Equipment
4.3. Synthesis of H2-L, H2-χ-L, Ni-L and Ni-χ-L
4.4. Association Constant and Detection Limit
4.5. pH Titration
4.6. Quantum Yield (QYs) Measurement
4.7. The pH Response of Different Metals
4.8. Stability and Reversibility
4.9. Computational Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| H2-χ-L | 3:5-Cl-saldmpn = N, N’-( 3,3′-dipropyhnethylamine) bis (3,5-chlorosalicylidene) |
| Ni-χ-L | NiII(3,5-Cl-saldmpn) |
| H2-L | saldmpn = N, N’-( 3,3′-dipropyhnethylamine) bis (salicylideneiminato) |
| Ni-L | NiII (saldmpn) |
References
- Huang, M.; Lu, H.; Wang, K.; Liu, B.; Yang, J. A facile design of azaanthracene derivatives: Acq–aie conversion and blue-shifted mechanofluorochromic emission. Dye. Pigment. 2020, 186, 108992. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Gaussian, Inc. Wallingford CT 2009, 32, 5648–5652. [Google Scholar]
- Ruiyun, C.; Ian, R.; Peter, A.W.; Shunjing, L.; Jun, C.; Chengmei, L. The influence of pH and monovalent ions on the gelation of pectin from the fruit seeds of the creeping fig plant. Food Hydrocoll. 2021, 111, 106219. [Google Scholar]
- Xu, G.K.; Feng, X.Q.; Li, B.; Gao, H. Controlled Release and Assembly of Drug Nanoparticles via pH-Responsive Polymeric Micelles: A Theoretical Study. J. Phys. Chem. B 2012, 116, 6003. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, D.W.; Song, J.L.; Sun, X.W.; Deng, W.Q.; Liu, X.W.; Lei, W. Directly assembled CdSe quantum dots on TiO2 in aqueous solution by adjusting pH value for quantum dot sensitized solar cells. Electrochem. Commun. 2009, 11, 2265–2267. [Google Scholar] [CrossRef]
- Sun, H.; Bai, Y.; Liu, H.; Jin, W.; Xu, N.; Chen, G.; Xu, B. Mechanism of Nitrogen-Concentration Dependence on pH Value: Experimental and Theoretical Studies on Nitrogen-Doped TiO2. J. Phys. Chem. C 2008, 112, 13304–13309. [Google Scholar] [CrossRef]
- Andreas, S.; Otto, S.W.; Sergey, M.B. Optical Sensing and Imaging of pH Values: Spectroscopies, Materials, and Applications. Chem. Rev. 2020, 120, 12357–12489. [Google Scholar]
- Ferrari, L.; Rovati, L.; Fabbri, P.; Pilati, F. Disposable Fluorescence Optical pH Sensor for Near Neutral Solutions. Sensors 2013, 13, 484–499. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.-G.; Gui, X.-Q.; Zeng, G.-M.; Yuan, X.-Z. A ratiometric fluorescence sensor with broad dynamic range based on two pH-sensitive fluorophores. Analyst 2005, 130, 1551–1556. [Google Scholar] [CrossRef]
- Yan, W.; Liyuan, Z.; Aojie, X.; Lei, W.; Lingli, Z.; Shuangqiang, L.; Yongjun, L.; Hanyang, L. Detecting enzymatic reactions in penicillinase via liquid crystal microdroplet-based pH sensor. Sens. Actuators B Chem. 2017, 258, 1090–1098. [Google Scholar]
- Yue, Y.; Huo, F.; Lee, S.; Yin, C.; Yoon, J. A review: The trend of progress about pH probes in cell application in recent years. Analyst 2017, 142, 30–41. [Google Scholar] [CrossRef]
- El-Mahdy, A.F.; Lai, M.-Y.; Kuo, S.-W. A highly fluorescent covalent organic framework as a hydrogen chloride sensor: Roles of Schiff base bonding and π-stacking. J. Mater. Chem. C 2020, 8, 9520–9528. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, K.; Hu, X.; Shi, P.; Guo, Z.; Zhan, H. Novel One-Dimensional Covalent Organic Framework as a H+ Fluorescent Sensor in Acidic Aqueous Solution. ACS Appl. Mater. Interfaces 2020, 13, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Guo, H.; Wang, T.; Gong, L.; Wang, Y.; Ai, J.; Huang, D.; Chen, H.; Yang, W. Fluorescence properties and analytical applications of covalent organic frameworks. Anal. Methods 2017, 9, 3737–3750. [Google Scholar] [CrossRef]
- Huang, L.; Chen, Y.; Liang, B.; Xing, B.; Wen, G.; Wang, S.; Yue, X.; Zhu, C.; Du, J.; Bu, X. A furanyl acryl conjugated coumarin as an efficient inhibitor and a highly selective off–on fluorescent probe for covalent labelling of thioredoxin reductase. Chem. Commun. 2014, 50, 6987–6990. [Google Scholar] [CrossRef]
- Fukuhara, G. Analytical supramolecular chemistry: Colorimetric and fluorimetric chemosensors. J. Photochem. Photobiol. C Photochem. Rev. 2020, 42, 100340. [Google Scholar] [CrossRef]
- Shi, M.W.; Thomas, S.P.; Koutsantonis, G.A.; Spackman, M.A. Supramolecular Recognition and Energy Frameworks in Host–Guest Complexes of 18-Crown-6 and Sulfonamides. Cryst. Growth Des. 2015, 15, 5892–5900. [Google Scholar] [CrossRef]
- Wang, M.; Li, Q.; Li, E.; Liu, J.; Huang, F. Vapochromic Behaviors of A Solid-State Supramolecular Polymer Based on Exo-wall Complexation of Perethylated Pillar[5]arene with 1,2,4,5-Tetracyanobenzene. Angew. Chem. Int. Ed. 2021, 60, 8115–8120. [Google Scholar] [CrossRef]
- Huang, F.; Anslyn, E.V. Introduction: Supramolecular Chemistry. Chem. Rev. 2015, 115, 6999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slater, A.G.; Perdigão, L.M.A.; Beton, P.H.; Champness, N.R. Surface-Based Supramolecular Chemistry Using Hydrogen Bonds. Acc. Chem. Res. 2014, 47, 3417–3427. [Google Scholar] [CrossRef]
- Li, B.; Zang, S.Q.; Wang, L.Y.; Mak, T.C.W. Halogen bonding: A powerful, emerging tool for constructing high-dimensional metal-containing supramolecular networks. Coord. Chem. Rev. 2016, 308, 1–21. [Google Scholar] [CrossRef]
- Appel, E.A.; Del Barrio, J.; Loh, X.J.; Scherman, O.A. Supramolecular polymeric hydrogels. Chem. Soc. Rev. 2012, 41, 6195–6214. [Google Scholar] [CrossRef]
- Gilday, L.C.; Robinson, S.W.; Barendt, T.A.; Langton, M.J.; Mullaney, B.R.; Beer, P.D. Halogen Bonding in Supramolecular Chemistry. Angew. Chem. Int. Ed. 2010, 47, 6114–6127. [Google Scholar] [CrossRef]
- Thomas Iii, S.W.; Joly, G.D.; Swager, T.M. Chemical sensors based on amplifying fluorescent conjugated polymers. Chem. Rev. 2007, 107, 1339–1386. [Google Scholar] [CrossRef] [PubMed]
- Birks, J.B. Photophysics of Aromatic Molecules; Wiley-Interscience: Hoboken, NJ, USA, 1970. [Google Scholar]
- Huang, M.; Yu, R.; Xu, K.; Ye, S.; Kuang, S.; Zhu, X.; Wan, Y. An arch-bridge-type fluorophore for bridging the gap between aggregation-caused quenching (ACQ) and aggregation-induced emission (AIE). Chem. Sci. 2016, 7, 4485–4491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Sheng, X.; Zebing, Z.; Ben Zhong, T. Functional Scaffolds from AIE Building Blocks. Matter 2020, 3, 1862–1892. [Google Scholar]
- Würthner, F. Aggregation-Induced Emission (AIE): A Historical Perspective. Angew. Chem. Int. Ed. 2020, 59, 14192–14196. [Google Scholar] [CrossRef]
- Melendez-Zamudio, M.; Guerra-Contreras, A.; Villegas, A.; Cervantes, J. Aggregation Induced Emission (AIE) Effect Based on Fluorescent Amino–Siloxane Copolymers. J. Inorg. Organomet. Polym. Mater. 2020, 30, 994–1001. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 1740–1741. [Google Scholar] [CrossRef] [PubMed]
- Galán, L.A.; Cordes, D.B.; Slawin, A.M.Z.; Jacquemin, D.; Ogden, M.I.; Massi, M.; Zysman-Colman, E. Analyzing the Relation between Structure and Aggregation Induced Emission (AIE) Properties of Iridium(III) Complexes through Modification of Non-Chromophoric Ancillary Ligands. Eur. J. Inorg. Chem. 2019, 2019, 152–163. [Google Scholar] [CrossRef] [Green Version]
- Segura, J.L.; Mancheño, M.J.; Zamora, F. Covalent organic frameworks based on Schiff-base chemistry: Synthesis, properties and potential applications. Chem. Soc. Rev. 2016, 45, 5635–5671. [Google Scholar] [CrossRef] [PubMed]
- Leovac, V.M.; Jevtović, V.S.; Jovanović, L.S.; Bogdanović, G.A. Metal complexes with Schiff-base ligands—pyridoxal and semicarbazide-based derivatives. J. Serb. Chem. Soc. 2005, 70, 393–422. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, Y.; Ma, X.; Zhou, X.; Xiang, H. Colorimetric and fluorescent pH and Cu2+ probes induced by photoisomerization of a maleonitrile-based Salen ligand. Chem. Commun. 2013, 49, 11791–11793. [Google Scholar] [CrossRef]
- Pan, Y.Q.; Xu, X.; Zhang, Y.; Zhang, Y.; Dong, W.K. A highly sensitive and selective bis(salamo)-type fluorescent chemosensor for identification of Cu2+ and the continuous recognition of S2−, Arginine and Lysine. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2020, 229, 117927. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wei, Z.L.; Mu, H.R.; Dong, W.K.; Ding, Y.J. A novel unsymmetric bis(salamo)-based chemosensor for detecting Cu2+ and continuous recognition of amino acids. J. Photochem. Photobiol. A Chem. 2020, 397, 112569. [Google Scholar] [CrossRef]
- Shoora, S.K.; Jain, A.K.; Gupta, V.K. A simple Schiff base based novel optical probe for aluminium (III) ions. Sens. Actuators B Chem. 2015, 216, 86–104. [Google Scholar] [CrossRef]
- Dalapati, S.; Jana, S.; Guchhait, N. Anion recognition by simple chromogenic and chromo-fluorogenic salicylidene Schiff base or reduced-Schiff base receptors. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 129, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.W.; Park, G.J.; Na, Y.J.; Jo, H.Y.; Lee, S.A.; You, G.R.; Kim, C. A single schiff base molecule for recognizing multiple metal ions: A fluorescence sensor for Zn(II) and Al(III) and colorimetric sensor for Fe(II) and Fe(III). Sens. Actuators B Chem. 2014, 194, 343–352. [Google Scholar] [CrossRef]
- Onami, Y.; Kawasaki, T.; Aizawa, H.; Haraguchi, T.; Akitsu, T.; Tsukiyama, K.; Palafox, M.A. Degradation of human serum albumin by infrared free electron laser enhanced by inclusion of a salen-type Schiff base Zn (II) complex. Int. J. Mol. Sci. 2020, 21, 874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhary, A.; Das, B.; Ray, S. Encapsulation of a Ni salen complex in zeolite Y: An experimental and DFT study. Dalton Trans. 2015, 44, 3753–3763. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, H.P.; Hadi, J.S.; Abdulnabi, Z.A.; Bolandnazar, Z. Spectroscopic, thermal analysis and DFT computational studies of salen-type Schiff base complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 485–492. [Google Scholar] [CrossRef]
- Wang, L.; Wei, Z.-L.; Chen, Z.-Z.; Liu, C.; Dong, W.-K.; Ding, Y.-J. A chemical probe capable for fluorescent and colorimetric detection to Cu2+ and CN− based on coordination and nucleophilic addition mechanism. Microchem. J. 2020, 155, 104801. [Google Scholar] [CrossRef]
- Zhang, F.; Dong, W.; Ma, Y.; Jiang, T.; Liu, B.; Li, X.; Shao, Y.; Wu, J. Fluorescent pH probes for alkaline pH range based on perylene tetra-(alkoxycarbonyl) derivatives. Arab. J. Chem. 2020, 13, 5900–5910. [Google Scholar] [CrossRef]
- Franks, M.; Gadzhieva, A.; Ghandhi, L.; Murrell, D.; Blake, A.J.; Davies, E.S.; Lewis, W.; Moro, F.; McMaster, J.; Schröder, M. Five Coordinate M(II)-Diphenolate [M = Zn(II), Ni(II), and Cu(II)] Schiff Base Complexes Exhibiting Metal- and Ligand-Based Redox Chemistry. Inorg. Chem. 2013, 52, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glendening, E.D.; Landis, C.R.; Weinhold, F. Natural bond orbital methods. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 1–42. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Murray, J.S.; Politzer, P. The electrostatic potential: An overview. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2011, 1, 153–163. [Google Scholar] [CrossRef]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2010, 42, 339–341. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Pang, H.; Wu, Q.; Huang, M.; Xu, J.; Zheng, L.; Wang, B.; Qiao, Y. Rigid Schiff Base Complex Supermolecular Aggregates as a High-Performance pH Probe: Study on the Enhancement of the Aggregation-Caused Quenching (ACQ) Effect via the Substitution of Halogen Atoms. Int. J. Mol. Sci. 2022, 23, 6259. https://doi.org/10.3390/ijms23116259
Li T, Pang H, Wu Q, Huang M, Xu J, Zheng L, Wang B, Qiao Y. Rigid Schiff Base Complex Supermolecular Aggregates as a High-Performance pH Probe: Study on the Enhancement of the Aggregation-Caused Quenching (ACQ) Effect via the Substitution of Halogen Atoms. International Journal of Molecular Sciences. 2022; 23(11):6259. https://doi.org/10.3390/ijms23116259
Chicago/Turabian StyleLi, Tianyu, Haijun Pang, Qiong Wu, Meifen Huang, Jiajun Xu, Liping Zheng, Baoling Wang, and Yongfeng Qiao. 2022. "Rigid Schiff Base Complex Supermolecular Aggregates as a High-Performance pH Probe: Study on the Enhancement of the Aggregation-Caused Quenching (ACQ) Effect via the Substitution of Halogen Atoms" International Journal of Molecular Sciences 23, no. 11: 6259. https://doi.org/10.3390/ijms23116259