Graphene for Antimicrobial and Coating Application
Abstract
:1. Introduction
2. Production of Graphene
3. Structure and Properties of Graphene
4. Characterization and Properties of Graphene
5. Functionalization of Graphene
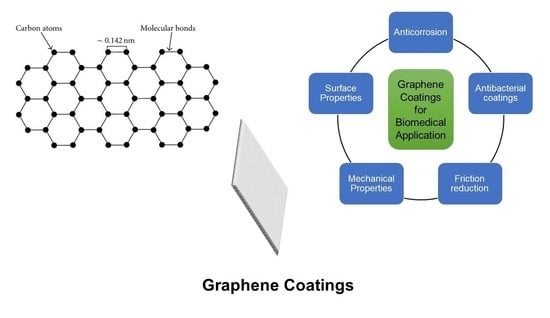
6. Graphene Coating Applications
6.1. Anticorrosion Coating
6.2. Antibacterial Application
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Katsnelson, M.I. Graphene: Carbon in two dimensions. Mater. Today 2007, 10, 20–27. [Google Scholar] [CrossRef]
- Kuill, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene-based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Samulski, E.T. Synthesis of water-soluble graphene. Nano Lett. 2008, 8, 1679–1682. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Dubey, N.; Bentini, R.; Islam, I.; Cao, T.; Castro Neto, A.H.; Rosa, V. Graphene: A versatile carbon-based material for bone tissue engineering. Stem. Cells Int. 2015, 2015, 804213. [Google Scholar] [CrossRef]
- Rosa, V.; Zhang, Z.; Grande, R.H.; Nor, J.E. Dental pulp tissue engineering in full-length human root canals. J. Dent. Res. 2013, 92, 970–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef]
- Mao, H.Y.; Laurent, S.; Chen, W.; Akhavan, O.; Imani, M.; Ashkarran, A.A.; Mahmoudi, M. Graphene: Promises, facts, opportunities, and challenges in nanomedicine. Chem. Rev. 2013, 113, 3407–3424. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, L.; Liu, M.; Zhang, Z. Biomedical applications of graphene. Theranostics 2012, 2, 283–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, M.; Clemons, C.; Wilber, J.; Young, G.; Buldum, A.; Quinn, D. Continuum plate theory and atomistic modeling to find the flexural rigidity of a graphene sheet interacting with a substrate. J. Nanotechnol. 2010, 2010, 868492. [Google Scholar] [CrossRef] [Green Version]
- Al-Sherbini, A.-S.; Bakr, M.; Ghoneim, I.; Saad, M. Exfoliation of graphene sheets via high energy wet milling of graphite in 2-ethylhexanol and kerosene. J. Adv. Res. 2017, 8, 209–215. [Google Scholar] [CrossRef]
- Geim, A.K. Nobel lecture: Random walk to graphene. Rev. Mod. Phys. 2011, 83, 851–862. [Google Scholar] [CrossRef] [Green Version]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Guy, O.J.; Walker, K.-A.D. Chapter 4—Graphene functionalization for biosensor applications. In Silicon Carbide Biotechnology, 2nd ed.; Saddow, S.E., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 85–141. [Google Scholar]
- Michon, A.; Vézian, S.; Ouerghi, A.; Zielinski, M.; Chassagne, T.; Portail, M. Direct growth of few-layer graphene on 6h-sic and 3c-sic/si via propane chemical vapor deposition. Appl. Phys. Lett. 2010, 97, 171909. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.-B.; Xie, W.; Du, J. Quantitative analysis of graphene doping by organic molecular charge transfer. J. Phys. Chem. C 2011, 115, 7596–7602. [Google Scholar] [CrossRef]
- Järvinen, P.; Hämäläinen, S.K.; Banerjee, K.; Häkkinen, P.; Ijäs, M.; Harju, A.; Liljeroth, P. Molecular self-assembly on graphene on SiO2 and h-bn substrates. Nano Lett. 2013, 13, 3199–3204. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.H.; Hersam, M.C. Room-temperature molecular-resolution characterization of self-assembled organic monolayers on epitaxial graphene. Nat. Chem. 2009, 1, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Cao, H.; Xue, Y.; Li, B.; Cai, W. Liquid-phase exfoliation of graphene: An overview on exfoliation media, techniques, and challenges. Nanomaterials 2018, 8, 942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paton, K.R.; Varrla, E.; Backes, C.; Smith, R.J.; Khan, U.; O’Neill, A.; Boland, C.; Lotya, M.; Istrate, O.M.; King, P.; et al. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nat. Mater. 2014, 13, 624–630. [Google Scholar] [CrossRef]
- Ciesielski, A.; Samori, P. Graphene via sonication assisted liquid-phase exfoliation. Chem. Soc. Rev. 2014, 43, 381–398. [Google Scholar] [CrossRef]
- Shen, Z.; Li, J.; Yi, M.; Zhang, X.; Ma, S. Preparation of graphene by jet cavitation. Nanotechnology 2011, 22, 365306. [Google Scholar] [CrossRef]
- Karagiannidis, P.G.; Hodge, S.A.; Lombardi, L.; Tomarchio, F.; Decorde, N.; Milana, S.; Goykhman, I.; Su, Y.; Mesite, S.V.; Johnstone, D.N.; et al. Microfluidization of graphite and formulation of graphene-based conductive inks. ACS Nano 2017, 11, 2742–2755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lotya, M.; King, P.J.; Khan, U.; De, S.; Coleman, J.N. High-concentration, surfactant-stabilized graphene dispersions. ACS Nano 2010, 4, 3155–3162. [Google Scholar] [CrossRef]
- Han, J.T.; Jang, J.I.; Kim, H.; Hwang, J.Y.; Yoo, H.K.; Woo, J.S.; Choi, S.; Kim, H.Y.; Jeong, H.J.; Jeong, S.Y.; et al. Extremely efficient liquid exfoliation and dispersion of layered materials by unusual acoustic cavitation. Sci. Rep. 2014, 4, 5133. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, A.S.; Obraztsova, E.A.; Belkin, A.V.; Monat, C.; Rojo-Romeo, P.; Obraztsova, E.D. Liquid-phase exfoliation of flaky graphite. J. Nanophotonics 2016, 10, 012525. [Google Scholar] [CrossRef]
- Lin, Z.; Karthik, P.S.; Hada, M.; Nishikawa, T.; Hayashi, Y. Simple technique of exfoliation and dispersion of multilayer graphene from natural graphite by ozone-assisted sonication. Nanomaterials 2017, 7, 125. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Yu, G. Direct cvd graphene growth on semiconductors and dielectrics for transfer-free device fabrication. Adv. Mater. 2016, 28, 4956–4975. [Google Scholar] [CrossRef]
- Teng, P.Y.; Lu, C.C.; Akiyama-Hasegawa, K.; Lin, Y.C.; Yeh, C.H.; Suenaga, K.; Chiu, P.W. Remote catalyzation for direct formation of graphene layers on oxides. Nano Lett. 2012, 12, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Shi, L.; Zhang, Y.; Liu, Z. Scalable chemical-vapor-deposition growth of three-dimensional graphene materials towards energy-related applications. Chem. Soc. Rev. 2018, 47, 3018–3036. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Chai, Z.; Li, C.; Shi, L.; Liu, M.; Xie, Q.; Zhang, Y.; Xu, D.; Manivannan, A.; Liu, Z. Catalyst-free growth of three-dimensional graphene flakes and graphene/g-c3n4 composite for hydrocarbon oxidation. ACS Nano 2016, 10, 3665–3673. [Google Scholar] [CrossRef]
- Wang, H.; Sun, K.; Tao, F.; Stacchiola, D.J.; Hu, Y.H. 3D honeycomb-like structured graphene and its high efficiency as a counter-electrode catalyst for dye-sensitized solar cells. Angew. Chem. Int. Ed. Engl. 2013, 52, 9210–9214. [Google Scholar] [CrossRef] [Green Version]
- Rokaya, D.; Srimaneepong, V.; Thunyakitpisal, P.; Qin, J.; Rosa, V.; Sapkota, J. Potential applications of graphene-based nanomaterials in biomedical, dental, and implant applications. In Advances in Dental Implantology Using Nanomaterials and Allied Technology Applications; Chaughule, R.S., Dashaputra, R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 77–105. [Google Scholar]
- Gupta, A.; Chen, G.; Joshi, P.; Tadigadapa, S.; Eklund, P.C. Raman scattering from high-frequency phonons in supported n-graphene layer films. Nano Lett. 2006, 6, 2667–2673. [Google Scholar] [CrossRef] [Green Version]
- Shearer, C.J.; Slattery, A.D.; Stapleton, A.J.; Shapter, J.G.; Gibson, C.T. Accurate thickness measurement of graphene. Nanotechnology 2016, 27, 125704. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; De Hosson, J.T.M.; Peia, Y. Three-dimensional micron-porous graphene foams for lightweight current collectors of lithium-sulfur batteries. Carbon 2019, 144, 713–723. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Mohanty, S.; Mukherjee, S.; Basu, S.; Mishra, M. Graphene and graphene oxide as nanomaterials for medicine and biology application. J. Nanostruct. Chem. 2018, 8, 123–137. [Google Scholar] [CrossRef] [Green Version]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Kovtyukhova, N.I.; Ollivier, P.J.; Martin, B.R.; Mallouk, T.E.; Chizhik, S.A.; Buzaneva, E.V.; Gorchinskiy, A.D. Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem. Mater. 1999, 11, 771–778. [Google Scholar] [CrossRef]
- Sang Tran, T.; Dutta, N.K.; Roy Choudhury, N. Graphene-based inks for printing of planar micro-supercapacitors: A review. Materials 2019, 12, 978. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Pandit, S.; Fu, Y.; Mijakovic, I.; Jesorka, A.; Liu, J. Graphene oxide-based coatings on nitinol for biomedical implant applications: Effectively promote mammalian cell growth but kill bacteria. RSC Adv. 2016, 6, 38124–38134. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Ryzhkov, S.A.; Kirilenko, D.A.; Ulin, N.V.; Baidakova, M.V.; Shnitov, V.V.; Pavlov, S.I.; Chumakov, R.G.; Stolyarova, D.Y.; Besedina, N.A.; et al. From graphene oxide towards aminated graphene: Facile synthesis, its structure, and electronic properties. Sci. Rep. 2020, 10, 6902. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- de Faria, A.F.; Martinez, D.S.T.; Meira, S.M.M.; de Moraes, A.C.M.; Brandelli, A.; Filho, A.G.S.; Alves, O.L. Anti-adhesion and antibacterial activity of silver nanoparticles supported on graphene oxide sheets. Colloids Surf. B 2014, 113, 115–124. [Google Scholar] [CrossRef]
- Graf, D.; Molitor, F.; Ensslin, K.; Stampfer, C.; Jungen, A.; Hierold, C.; Wirtz, L. Spatially resolved raman spectroscopy of single- and few-layer graphene. Nano Lett. 2007, 7, 238–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Wang, L.; Chen, G. Modification of graphene platelets and their tribological properties as a lubricant additive. Tribol. Lett. 2011, 41, 209–215. [Google Scholar] [CrossRef]
- Park, J.H.; Park, J.M. Electrophoretic deposition of graphene oxide on mild carbon steel for anti-corrosion application. Surf. Coat. Technol. 2014, 254, 167–174. [Google Scholar] [CrossRef]
- Hao, J.; Ji, L.; Wu, K.; Yang, N. Electrochemistry of zno@reduced graphene oxides. Carbon 2018, 130, 480–486. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, X.; Wang, H.; Zheng, W. Graphene oxide-ag nanocomposite: In situ photochemical synthesis and application as a surface-enhanced raman scattering substrate. Thin Solid Films 2011, 520, 179–185. [Google Scholar] [CrossRef]
- Shao, W.; Liu, X.L.; Min, H.; Dong, G.; Feng, Q.; Zuo, S. Preparation, characterization, and antibacterial activity of silver nanoparticle-decorated graphene oxide nanocomposite. ACS Appl. Mater Interfaces 2015, 7, 6966–6973. [Google Scholar] [CrossRef]
- Banerjee, A.N. Graphene and its derivatives as biomedical materials: Future prospects and challenges. Interface Focus 2018, 8, 20170056. [Google Scholar] [CrossRef]
- Usachov, D.; Vilkov, O.; Gruneis, A.; Haberer, D.; Fedorov, A.; Adamchuk, V.K.; Preobrajenski, A.B.; Dudin, P.; Barinov, A.; Oehzelt, M.; et al. Nitrogen-doped graphene: Efficient growth, structure, and electronic properties. Nano Lett. 2011, 11, 5401–5407. [Google Scholar] [CrossRef]
- Hoik, L.; Keewook, P.; Ick, S.K. A review of doping modulation in graphene. Synth. Met. 2018, 244, 36–47. [Google Scholar]
- Wang, L.; Yang, Z.; Cui, Y.; Wei, B.; Xu, S.; Sheng, J.; Wang, M.; Zhu, Y.; Fei, W. Graphene-copper composite with micro-layered grains and ultrahigh strength. Sci. Rep. 2017, 7, 41896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rokaya, D.; Srimaneepong, V.; Qin, J.; Siraleartmukul, K.; Siriwongrungson, V. Graphene oxide/silver nanoparticles coating produced by electrophoretic deposition improved the mechanical and tribological properties of niti alloy for biomedical applications. J. Nanosci. Nanotechnol. 2018, 18, 3804–3810. [Google Scholar] [CrossRef]
- Rokaya, D.; Srimaneepong, V.; Sapkota, J.; Qin, J.; Siraleartmukul, K.; Siriwongrungson, V. Polymeric materials and films in dentistry: An overview. J. Adv. Res. 2018, 14, 25–34. [Google Scholar] [CrossRef]
- Ramanathan, T.; Abdala, A.A.; Stankovich, S.; Dikin, D.A.; Herrera-Alonso, M.; Piner, R.D.; Adamson, D.H.; Schniepp, H.C.; Chen, X.; Ruoff, R.S.; et al. Functionalized graphene sheets for polymer nanocomposites. Nat. Nanotechnol. 2008, 3, 327–331. [Google Scholar] [CrossRef]
- Yoo, J.J.; Balakrishnan, K.; Huang, J.; Meunier, V.; Sumpter, B.G.; Srivastava, A.; Conway, M.; Reddy, A.L.; Yu, J.; Vajtai, R.; et al. Ultrathin planar graphene supercapacitors. Nano Lett. 2011, 11, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Fu, L.; Liu, N.; Liu, M.; Wang, Y.; Liu, Z. Synthesis of nitrogen-doped graphene using embedded carbon and nitrogen sources. Adv. Mater. 2011, 23, 1020–1024. [Google Scholar] [CrossRef]
- Yang, Y.; Asiri, A.M.; Tang, Z.; Du, D.; Lin, Y. Graphene based materials for biomedical applications. Mater. Today 2013, 16, 365–373. [Google Scholar] [CrossRef]
- Rokaya, D.; Srimaneepong, V.; Qin, J.; Thunyakitpisal, P.; Siraleartmukul, K. Surface adhesion properties and cytotoxicity of graphene oxide coatings and graphene oxide/silver nanocomposite coatings on biomedical niti alloy. Sci. Adv. Mater. 2019, 11, 1474–1487. [Google Scholar] [CrossRef]
- Tong, Y.; Bohm, S.; Song, M. Graphene based materials and their composites as coatings. Austin J. Nanomed. Nanotechnol. 2013, 1, 1003. [Google Scholar]
- Kirkland, N.T.; Schiller, T.; Medhekar, N.; Birbilis, N. Exploring graphene as a corrosion protection barrier. Corros. Sci. 2012, 56, 1–4. [Google Scholar] [CrossRef]
- Nam, J.A.; Nahain, A.-A.; Kim, S.M.; In, I.; Park, S.Y. Successful stabilization of functionalized hybrid graphene for high-performance antimicrobial activity. Acta. Biomater. 2013, 9, 7996–8003. [Google Scholar] [CrossRef]
- Johannsen, J.C.; Ulstrup, S.; Crepaldi, A.; Cilento, F.; Zacchigna, M.; Miwa, J.A.; Cacho, C.; Chapman, R.T.; Springate, E.; Fromm, F.; et al. Tunable carrier multiplication and cooling in graphene. Nano Lett. 2015, 15, 326–331. [Google Scholar] [CrossRef] [Green Version]
- Wei, D.; Liu, Y.; Wang, Y.; Zhang, H.; Huang, L.; Yu, G. Synthesis of n-doped graphene by chemical vapor deposition and its electrical properties. Nano Lett. 2009, 9, 1752–1758. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Bao, D.; Liu, H.; Zhang, D.; Wang, N.; Li, H. Functionalization of graphene and applications of the derivatives. J. Inorg. Organomet. Polym. 2017, 27, 1129–1141. [Google Scholar] [CrossRef]
- Sinitskii, A.; Dimiev, A.; Corley, D.A.; Fursina, A.A.; Kosynkin, D.V.; Tour, J.M. Kinetics of diazonium functionalization of chemically converted graphene nanoribbons. ACS Nano 2010, 4, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- Hetemi, D.; Noël, V.; Pinson, J. Grafting of diazonium salts on surfaces: Application to biosensors. Biosensors 2020, 10, 4. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.-H.; Yan, M. Simple method for the covalent immobilization of graphene. Nano Lett. 2009, 9, 3375–3378. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.-H.; Yan, M. Functionalization of pristine graphene with perfluorophenyl azides. J. Mater. Chem. 2011, 21, 3273–3276. [Google Scholar] [CrossRef]
- Chang, C.-H.; Fan, X.; Li, L.-J.; Kuo, J.-L. Band gap tuning of graphene by adsorption of aromatic molecules. J. Phys. Chem. C 2012, 116, 13788–13794. [Google Scholar] [CrossRef]
- Park, H.; Lee, J.Y.; Shin, S. Tuning of the band structures of zigzag graphene nanoribbons by an electric field and adsorption of pyridine and bf3: A dft study. J. Phys. Chem. C 2012, 116, 20054–20061. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Zhao, D.; Zeng, D.; Xia, J.; Aldalbahi, A.; Wang, C.; San, L.; Fan, C.; Zuo, X.; et al. Universal fluorescence biosensor platform based on graphene quantum dots and pyrene-functionalized molecular beacons for detection of micrornas. ACS Appl. Mater. Interfaces 2015, 7, 16152–16156. [Google Scholar] [CrossRef] [PubMed]
- Hui, K.S.; Hui, K.N.; Dinh, D.A.; Tsang, C.H.; Cho, Y.R.; Zhou, W.; Hong, X.; Chun, H.H. Green synthesis of dimension- controlled silver nanoparticle-graphene oxide with in situ ultrasonication. Acta Mater. 2014, 64, 326–332. [Google Scholar] [CrossRef]
- Jeyaseelan, A.; Ghfar, A.A.; Naushad, M.; Viswanathan, N. Design and synthesis of amine functionalized graphene oxide for enhanced fluoride removal. J. Environ. Chem. Eng. 2021, 9, 105384. [Google Scholar] [CrossRef]
- Shadjou, N.; Hasanzadeh, M. Graphene and its nanostructure derivatives for use in bone tissue engineering: Recent advances. J. Biomed. Mater. Res. Part A 2016, 104, 1250–1275. [Google Scholar] [CrossRef]
- Prasadh, S.; Suresh, S.; Wong, R. Osteogenic potential of graphene in bone tissue engineering scaffolds. Materials 2018, 11, 1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shadjou, N.; Hasanzadeh, M.; Khalilzadeh, B. Graphene based scaffolds on bone tissue engineering. Bioengineered 2018, 9, 38–47. [Google Scholar] [PubMed] [Green Version]
- Cheng, J.; Liu, J.; Wu, B.; Liu, Z.; Li, M.; Wang, X.; Tang, P.; Wang, Z. Graphene and its derivatives for bone tissue engineering: In vitro and in vivo evaluation of graphene-based scaffolds, membranes and coatings. Front. Bioeng. Biotechnol. 2021, 9, 734688. [Google Scholar] [CrossRef]
- Nishida, E.; Miyaji, H.; Takita, H.; Kanayama, I.; Tsuji, M.; Akasaka, T.; Sugaya, T.; Sakagami, R.; Kawanami, M. Graphene oxide coating facilitates the bioactivity of scaffold material for tissue engineering. Jpn. J. Appl. Phys. 2014, 53, 06JD04. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Park, J.B.; Lee, T.-J.; Ryu, S.; Bhang, S.H.; La, W.-G.; Noh, M.-K.; Hong, B.H.; Kim, B.-S. Covalent conjugation of mechanically stiff graphene oxide flakes to three-dimensional collagen scaffolds for osteogenic differentiation of human mesenchymal stem cells. Carbon 2015, 83, 162–172. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Yang, C.; Qi, X.; Li, S.; Liu, C.; Li, X. Mesoporous bioactive glass combined with graphene oxide scaffolds for bone repair. Int. J. Biol. Sci. 2019, 15, 2156–2169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skallevold, H.E.; Rokaya, D.; Khurshid, Z.; Zafar, M.S. Bioactive glass applications in dentistry. Int. J. Mol. Sci. 2019, 20, 5960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stepanova, M.; Solomakha, O.; Rabchinskii, M.; Averianov, I.; Gofman, I.; Nashchekina, Y.; Antonov, G.; Smirnov, A.; Ber, B.; Nashchekin, A.; et al. Aminated graphene-graft-oligo(glutamic acid)/poly(ε-caprolactone) composites: Preparation, characterization and biological evaluation. Polymers 2021, 13, 2628. [Google Scholar] [CrossRef]
- Podila, R.; Moore, T.; Alexis, F.; Rao, A. Graphene coatings for biomedical implants. J. Vis. Exp. 2013, 73, e50276. [Google Scholar] [CrossRef] [Green Version]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic biomaterials: Current challenges and opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef]
- McMahon, R.E.; Ma, J.; Verkhoturov, S.V.; Munoz-Pinto, D.; Karaman, I.; Rubitschek, F.; Maier, H.J.; Hahn, M.S. A comparative study of the cytotoxicity and corrosion resistance of nickel-titanium and titanium-niobium shape memory alloys. Acta Biomater. 2012, 8, 2863–2870. [Google Scholar] [CrossRef]
- Goryczka, T.; Szaraniec, B. Characterization of polylactide layer deposited on ni-ti shape memory alloy. J. Mater. Eng. Perform. 2014, 23, 2682–2686. [Google Scholar] [CrossRef] [Green Version]
- Williams, R.P.; Rinchuse, D.J.; Zullo, T.G. Perceptions of midline deviations among different facial types. Am. J. Orthod. Dentofac. Orthop. 2014, 145, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wu, G.; Xu, R.; Wang, W.; Wu, S.; Yeung, K.W.K.; Chu, P.K. In vitro corrosion inhibition on biomedical shape memory alloy by plasma-polymerized allylamine film. Mater. Lett. 2012, 89, 51–54. [Google Scholar] [CrossRef]
- Mazumder, M.M.; Mehta, J.L.; Mazumder, N.N.A.; Trigwell, S.; Sharma, R.; De, S. Encased Stent for Rapid Endothelialization for Preventing Restenosis. Patent 20040225346A1, 11 November 2004. [Google Scholar]
- Schellhammer, F.; Walter, M.; Berlis, A.; Bloss, H.-G.; Wellens, E.; Schumacher, M. Polyethylene terephthalate and polyurethane coatings for endovascular stents: Preliminary results in canine experimental arteriovenous fistulas. Radiology 1999, 211, 169–175. [Google Scholar] [CrossRef]
- Villermaux, F.; Tabrizian, M.; Yahia, L.H.; Czeremuszkin, G.; Piron, D.L. Corrosion resistance improvement of niti osteosynthesis staples by plasma polymerized tetrafiuoroethylene coating. Biomed. Mater. Eng. 1996, 6, 241–254. [Google Scholar] [PubMed]
- Tepe, G.; Schmehl, J.; Wendel, H.P.; Schaffner, S.; Heller, S.; Gianotti, M.; Claussen, C.D.; Duda, S.H. Reduced thrombogenicity of nitinol stents—in vitro evaluation of different surface modifications and coatings. Biomaterials 2006, 27, 643–650. [Google Scholar] [CrossRef]
- Anjum, S.S.; Rao, J.; Nicholls, J.R. Polymer (ptfe) and shape memory alloy (niti) intercalated nano-biocomposites. Mater. Sci. Eng. 2012, 40, 012006. [Google Scholar] [CrossRef] [Green Version]
- De Jesús, C.; Cruz, G.J.; Olayo, M.G.; Gómez, L.M.; López-Gracia, O.G. Coatings by plasmas of pyrrole on nitinol and stainless steel substrates. Superf. Vacío 2012, 25, 157–160. [Google Scholar]
- Yang, M.-R.; Wu, S.K. Dc plasma-polymerized hexamethyldisilazane coatings of an equiatomic tini shape memory alloy. Surf. Coat. Technol. 2000, 127, 274–281. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Dervishi, E.; Berry, B.; Viswanathan, T.; Bourdo, S.; Kim, H.; Sproles, R.; Hudson, M.K. Energy efficient graphite–polyurethane electrically conductive coatings for thermally actuated smart materials. Smart Mater. Struct. 2006, 15, S187. [Google Scholar] [CrossRef]
- Lin, W.S.; Metz, M.J.; Pollini, A.; Ntounis, A.; Morton, D. Digital data acquisition for a cad/cam-fabricated titanium framework and zirconium oxide restorations for an implant-supported fixed complete dental prosthesis. J. Prosthet. Dent. 2014, 112, 1324–1329. [Google Scholar] [CrossRef]
- Carroll, W.M.; Rochev, Y.; Clarke, B.; Burke, M.; Bradley, D.J.; Plumley, D.L. Influence of Nitinol Wire Surface Preparation Procedures, on Cell Surface Interactions and Polymer Coating Adherence, Materials & Processes for Medical Devices Conference, Anaheim, CA, USA, 8–10 September 2003; ASM International: Anaheim, CA, USA, 2004; pp. 63–68. [Google Scholar]
- Rokaya, D.; Srimaneepong, V.; Qin, J. Modification of titanium alloys for dental applications. In Metal, Metal Oxides and Metal Sulphides for Biomedical Applications; Rajendran, S., Naushad, M., Durgalakshmi, D., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 51–82. [Google Scholar]
- Raza, M.A.; Rehman, Z.U.; Ghauri, F.A.; Ahmad, A.; Ahmad, R.; Raffi, M. Corrosion study of electrophoretically deposited graphene oxide coatings on copper metal. Thin Solid Films 2016, 620, 150–159. [Google Scholar] [CrossRef]
- Nayak, P.K.; Hsu, C.-J.; Wang, S.-C.; Sung, J.C.; Huang, J.-L. Graphene coated ni films: A protective coating. Thin Solid Films 2013, 529, 312–316. [Google Scholar] [CrossRef]
- Catt, K.; Lia, H.; Cui, X.T. Poly (3,4-ethylenedioxythiophene) graphene oxide composite coatings for controlling magnesium implant corrosion. Acta. Biomater. 2017, 48, 530–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asgar, H.; Deen, K.M.; Rahman, Z.U.; Shah, U.H.; Raza, M.A.; Haider, W. Functionalized graphene oxide coating on ti6al4v alloy for improved biocompatibility and corrosion resistance. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 920–928. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, P.; Li, X.; Liu, H.; Ge, S. Bioactivity of periodontal ligament stem cells on sodium titanate coated with graphene oxide. Sci. Rep. 2016, 6, 19343. [Google Scholar] [CrossRef]
- Catt, K.; Li, H.; Hoang, V.; Beard, R.; Cui, X.T. Self-powered therapeutic release from conducting polymer/graphene oxide films on magnesium. Nanomedicine. 2018, 14, 2495–2503. [Google Scholar] [CrossRef]
- Singh, B.P.; Nayak, S.; Nanda, K.K.; Jena, B.K.; Bhattacharjee, S.; Besra, L. The production of a corrosion resistant graphene reinforced composite coating on copper by electrophoretic deposition. Carbon 2013, 61, 47–56. [Google Scholar] [CrossRef]
- Hikku, G.S.; Jeyasubramanian, K.; Venugopal, A.; Ghosh, R. Corrosion resistance behaviour of graphene/polyvinyl alcohol nanocomposite coating for aluminium-2219 alloy. J. Alloys Compd. 2017, 716, 259–269. [Google Scholar] [CrossRef]
- Suo, L.; Jiang, N.; Wang, Y.; Wang, P.; Chen, J.; Pei, X.; Wang, J.; Wan, Q. The enhancement of osseointegration using a graphene oxide/chitosan/hydroxyapatite composite coating on titanium fabricated by electrophoretic deposition. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 635–645. [Google Scholar] [CrossRef]
- Li, K.; Wang, C.; Yan, J.; Zhang, Q.; Dang, B.; Wang, Z.; Yao, Y.; Lin, K.; Guo, Z.; Bi, L.; et al. Evaluation of the osteogenesis and osseointegration of titanium alloys coated with graphene: An in vivo study. Sci. Rep. 2018, 8, 1843. [Google Scholar] [CrossRef] [Green Version]
- Rosa, V.; Rodríguez-Lozano, F.J.; Min, K. 22—Graphene to improve the physicomechanical properties and bioactivity of the cements. In Advanced Dental Biomaterials; Khurshid, Z., Najeeb, S., Zafar, M.S., Sefat, F., Eds.; Woodhead Publishing: Soston, UK, 2019; pp. 599–614. [Google Scholar]
- Nair, M.; Nancy, D.; Krishnan, A.G.; Anjusree, G.S.; Vadukumpully, S.; Nair, S.V. Graphene oxide nanoflakes incorporated gelatin-hydroxyapatite scaffolds enhance osteogenic differentiation of human mesenchymal stem cells. Nanotechnology 2015, 26, 161001. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, J.; Li, H. Synthesis of hydroxyapatite-reduced graphite oxide nanocomposites for biomedical applications: Oriented nucleation and epitaxial growth of hydroxyapatite. J. Mater. Chem. B 2013, 1, 1826–1834. [Google Scholar] [CrossRef]
- Xie, H.; Chua, M.; Islam, I.; Bentini, R.; Cao, T.; Viana-Gomes, J.C.; Castro Neto, A.H.; Rosa, V. Cvd-grown monolayer graphene induces osteogenic but not odontoblastic differentiation of dental pulp stem cells. Dent. Mater. 2017, 33, e13–e21. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Cao, T.; Gomes, J.V.; Castro Neto, A.H.; Rosa, V. Two and three-dimensional graphene substrates to magnify osteogenic differentiation of periodontal ligament stem cells. Carbon 2015, 93, 266–275. [Google Scholar] [CrossRef]
- Nishida, E.; Miyaji, H.; Kato, A.; Takita, H.; Iwanaga, T.; Momose, T.; Ogawa, K.; Murakami, S.; Sugaya, T.; Kawanami, M. Graphene oxide scaffold accelerates cellular proliferative response and alveolar bone healing of tooth extraction socket. Int. J. Nanomed. 2016, 11, 2265–2277. [Google Scholar]
- Høiby, N.; Ciofu, O.; Johansen, H.K.; Song, Z.-j.; Moser, C.; Jensen, P.Ø.; Molin, S.; Givskov, M.; Tolker-Nielsen, T.; Bjarnsholt, T. The clinical impact of bacterial biofilms. Int. J. Oral Sci. 2011, 3, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Dubey, N.; Ellepola, K.; Decroix, F.E.D.; Morin, J.L.P.; Castro Neto, A.H.; Seneviratne, C.J.; Rosa, V. Graphene onto medical grade titanium: An atom-thick multimodal coating that promotes osteoblast maturation and inhibits biofilm formation from distinct species. Nanotoxicology 2018, 12, 274–289. [Google Scholar] [CrossRef]
- Raphel, J.; Holodniy, M.; Goodman, S.B.; Heilshorn, S.C. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials 2016, 84, 301–314. [Google Scholar] [CrossRef] [Green Version]
- Gallo, J.; Holinka, M.; Moucha, C.S. Antibacterial surface treatment for orthopaedic implants. Int. J. Mol. Sci. 2014, 15, 13849–13880. [Google Scholar] [CrossRef] [Green Version]
- Smeets, R.; Henningsen, A.; Jung, O.; Heiland, M.; Hammächer, C.; Stein, J.M. Definition, etiology, prevention and treatment of peri-implantitis—A review. Head Face Med. 2014, 10, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monje, A.; Insua, A.; Wang, H.-L. Understanding peri-implantitis as a plaque-associated and site-specific entity: On the local predisposing factors. J. Clin. Med. 2019, 8, 279. [Google Scholar] [CrossRef] [Green Version]
- Manor, Y.; Oubaid, S.; Mardinger, O.; Chaushu, G.; Nissan, J. Characteristics of early versus late implant failure: A retrospective study. J. Oral Maxillofac. Surg. 2009, 67, 2649–2652. [Google Scholar] [CrossRef] [PubMed]
- Fragkioudakis, I.; Tseleki, G.; Doufexi, A.E.; Sakellari, D. Current concepts on the pathogenesis of peri-implantitis: A narrative review. Eur. J. Dent. 2021, 15, 379–387. [Google Scholar] [CrossRef]
- Rokaya, D.; Srimaneepong, V.; Wisitrasameewon, W.; Humagain, M.; Thunyakitpisal, P. Peri-implantitis update: Risk indicators, diagnosis, and treatment. Eur. J. Dent. 2020, 14, 672–682. [Google Scholar] [CrossRef]
- Szunerits, S.; Boukherroub, R. Antibacterial activity of graphene-based materials. J. Mater. Chem. B 2016, 4, 6892–6912. [Google Scholar] [CrossRef] [Green Version]
- Yañez-Macías, R.; Muñoz-Bonilla, A.; De Jesús-Tellez, M.A.; Maldonado-Textle, H.; Guerrero-Sánchez, C.; Schubert, U.S.; Guerrero-Santos, R. Combinations of antimicrobial polymers with nanomaterials and bioactives to improve biocidal therapies. Polymers 2019, 11, 1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sierra-Fernandez, A.; De la Rosa-García, S.C.; Yañez-Macías, R.; Guerrero-Sanchez, C.; Gomez-Villalba, L.S.; Gómez-Cornelio, S.; Rabanal, M.E.; Schubert, U.S.; Fort, R.; Quintana, P. Sol–gel synthesis of Mg(OH)2 and Ca(OH)2 nanoparticles: A comparative study of their antifungal activity in partially quaternized p(dmaema) nanocomposite films. J. Sol-Gel Sci. Technol. 2019, 89, 310–321. [Google Scholar] [CrossRef]
- Wong, K.K.Y.; Liu, X. Silver nanoparticles—The real “silver bullet” in clinical medicine? MedChemComm 2010, 1, 125–131. [Google Scholar] [CrossRef]
- Kong, H.; Jang, J. Antibacterial properties of novel poly(methyl methacrylate) nanofiber containing silver nanoparticles. Langmuir 2008, 24, 2051–2056. [Google Scholar] [CrossRef]
- Huang, C.C.; Chen, C.T.; Shiang, Y.C.; Lin, Z.H.; Chang, H.T. Synthesis of fluorescent carbohydrate-protected au nanodots for detection of concanavalin a and escherichia coli. Anal. Chem. 2009, 81, 875–882. [Google Scholar] [CrossRef]
- Senapati, T.; Senapati, D.; Singh, A.K.; Fan, Z.; Kanchanapally, R.; Ray, P.C. Highly selective sers probe for Hg(II) detection using tryptophan-protected popcorn shaped gold nanoparticles. Chem. Commun. 2011, 47, 10326–10328. [Google Scholar] [CrossRef] [PubMed]
- Agarwalla, S.V.; Ellepola, K.; Costa, M.; Fechine, G.J.M.; Morin, J.L.P.; Castro Neto, A.H.; Seneviratne, C.J.; Rosa, V. Hydrophobicity of graphene as a driving force for inhibiting biofilm formation of pathogenic bacteria and fungi. Dent. Mater. 2019, 35, 403–413. [Google Scholar] [CrossRef]
- Pipattanachat, S.; Qin, J.; Rokaya, D.; Thanyasrisung, P.; Srimaneepong, V. Biofilm inhibition and bactericidal activity of niti alloy coated with graphene oxide/silver nanoparticles via electrophoretic deposition. Sci. Rep. 2021, 11, 14008. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Lv, L.; Du, F.; Niu, T.; Chen, T.; Xia, D.; Wang, S.; Zhao, X.; Liu, J.; Liu, Y.; et al. Effects of thermal treatment on the adhesion strength and osteoinductive activity of single-layer graphene sheets on titanium substrates. Sci. Rep. 2018, 8, 8141. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Geng, H.; Zhu, H.; Zhang, M.; Di, Z.; Liu, X.; Chu, P.K.; Wang, X. Cvd growth of graphene on niti alloy for enhanced biological activity. ACS Appl. Mater. Interfaces 2015, 7, 19876–19881. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Peng, H.; Wang, X.; Shao, F.; Yuan, Z.; Han, H. Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale 2014, 6, 1879–1889. [Google Scholar] [CrossRef]








| Method | Crystallite Size (µm) | Sample Size (mm) | Charge Mobility (cm2 V−1 s−1) |
|---|---|---|---|
| CVD processed graphene | >1000 | ~1000 | 10,000 |
| Mechanical exfoliation of graphene | >1000 | >1 | >2 × 105 and 106 |
| Solution-processed graphene | ~100 | Infinite as a layer of graphene flakes | 100 |
| Epitaxial growth of graphene | 50 | 100 | 10,000 |
| Molecular assembly of graphene | <50 | >1 | NA |
| Properties | Graphene | GO | rGO |
|---|---|---|---|
| Thermal conductivity | 5000 W/m-K | 2000 W/m-K | 0.14–0.87 W/mK |
| Electrical conductivity | 104 S/cm | 10−1 S/cm | 200–35,000 S/cm |
| Electrical resistance | 10−6 Ω-cm | NA | NA |
| Tensile strength | 130 GPa | 120 GPa | NA |
| Elastic modulus | 1 TPa | 0.22 TPa | NA |
| Poisson’s ratio | 0.18 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srimaneepong, V.; Skallevold, H.E.; Khurshid, Z.; Zafar, M.S.; Rokaya, D.; Sapkota, J. Graphene for Antimicrobial and Coating Application. Int. J. Mol. Sci. 2022, 23, 499. https://doi.org/10.3390/ijms23010499
Srimaneepong V, Skallevold HE, Khurshid Z, Zafar MS, Rokaya D, Sapkota J. Graphene for Antimicrobial and Coating Application. International Journal of Molecular Sciences. 2022; 23(1):499. https://doi.org/10.3390/ijms23010499
Chicago/Turabian StyleSrimaneepong, Viritpon, Hans Erling Skallevold, Zohaib Khurshid, Muhammad Sohail Zafar, Dinesh Rokaya, and Janak Sapkota. 2022. "Graphene for Antimicrobial and Coating Application" International Journal of Molecular Sciences 23, no. 1: 499. https://doi.org/10.3390/ijms23010499