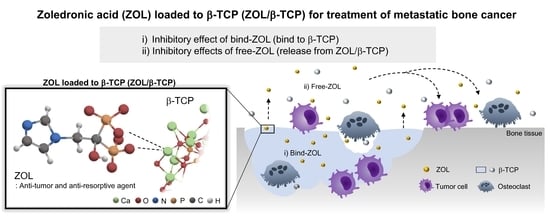
Zoledronic Acid-Loaded β-TCP Inhibits Tumor Proliferation and Osteoclast Activation: Development of a Functional Bone Substitute for an Efficient Osteosarcoma Treatment
Abstract
:1. Introduction
2. Results
2.1. Characterization of the ZOL-Loaded β-TCP
2.1.1. Adsorption of ZOL on β-TCP Powders
2.1.2. Zeta Potential
2.1.3. X-ray Diffractometry (XRD) Patterns
2.1.4. Solubility of Ca2+ from ZOL/β-TCP Powders
2.1.5. Release of ZOL from ZOL/β-TCP
2.2. Effects of ZOL/β-TCP on Osteoblasts and Tumor Cells
2.2.1. Anti-Tumor Effects
2.2.2. Cell Viability of Osteoblasts
2.3. Effects of ZOL/β-TCP on Osteoclast Differentiation and Activation
2.3.1. Inhibitory Effects for Osteoclast Formation and Activation on ZOL/β-TCP Disc
2.3.2. Effects of Free-ZOL on Osteoclast Activation
3. Discussion
4. Materials and Methods
4.1. Sample Fabrication and Characterization
4.1.1. Preparation of β-TCP Powders and Fabrication of Discs
4.1.2. Adsorption of Zoledronic acid to β-TCP Powders
4.1.3. X-ray Diffraction Analysis
4.1.4. Zeta Potential
4.1.5. Solubility of Ca2+ from ZOL/β-TCP Powders
4.1.6. Release of ZOL from ZOL/β-TCP Powders and ZOL/β-TCP Disc
4.2. Biological Evaluation
4.2.1. Cell Culture and Isolation
4.2.2. Preparation of Bone Slices
4.2.3. Preparation of Elution Medium
4.2.4. Cell Viability of MC3T3-E1 and HOS
4.2.5. Culture of mBMSCs on each ZOL/β-TCP Disc
4.2.6. Preparation of Osteoclastic Conditioned Medium
4.2.7. Culture of mBMSCs in an Osteoclastic Conditioned Medium
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mirabello, L.; Troisi, R.J.; Savage, S.A. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int. J. Cancer 2009, 125, 229–234. [Google Scholar] [CrossRef] [Green Version]
- Picci, P. Osteosarcoma (osteogenic sarcoma). Orphanet J. Rare Dis. 2007, 2, 6. [Google Scholar] [CrossRef] [Green Version]
- Ponzetti, M.; Rucci, N. Switching Homes: How Cancer Moves to Bone. Int. J. Mol. Sci. 2020, 21, 4124. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, H.; Dunstan, C.R.; Sutherland, R.L.; Seibel, M.J. The role of the bone microenvironment in skeletal metastasis. J. Bone Oncol. 2013, 2, 47–57. [Google Scholar] [CrossRef] [Green Version]
- Clines, G.A.; Guise, T.A. Molecular mechanisms and treatment of bone metastasis. Expert Rev. Mol. Med. 2008, 10. [Google Scholar] [CrossRef]
- Soeharno, H.; Povegliano, L.; Choong, P.F. Multimodal treatment of bone metastasis—A surgical perspective. Front. Endocrinol. 2018, 9, 518. [Google Scholar] [CrossRef] [PubMed]
- Meyers, P.A.; Heller, G.; Healey, J.H.; Huvos, A.; Applewhite, A.; Sun, M.; LaQuaglia, M. Osteogenic sarcoma with clinically detectable metastasis at initial presentation. J. Clin. Oncol. 1993, 11, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Chou, A.J.; Merola, P.R.; Wexler, L.H.; Gorlick, R.G.; Vyas, Y.M.; Healey, J.H.; LaQuaglia, M.P.; Huvos, A.G.; Meyers, P.A. Treatment of osteosarcoma at first recurrence after contemporary therapy: The Memorial Sloan-Kettering Cancer Center experience. Cancer Interdisciplinary Int. J. Am. Cancer Soc. 2005, 104, 2214–2221. [Google Scholar] [CrossRef] [PubMed]
- Van Beek, E.; Pieterman, E.; Cohen, L.; Löwik, C.; Papapoulos, S. Farnesyl pyrophosphate synthase is the molecular target of nitrogen-containing bisphosphonates. Biochem. Biophys. Res. Commun. 1999, 264, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.-T.; Cao, R.; Liang, P.-H.; Ko, T.-P.; Chang, T.-H.; Hudock, M.P.; Jeng, W.-Y.; Chen, C.K.-M.; Zhang, Y.; Song, Y. Bisphosphonates target multiple sites in both cis-and trans-prenyltransferases. Proc. Natl. Acad. Sci. USA 2007, 104, 10022–10027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleisch, H. Introduction to bisphosphonates. History and functional mechanisms. Der Orthop. 2007, 36, 103–104, 106. [Google Scholar] [CrossRef]
- Drake, M.T.; Clarke, B.L.; Khosla, S. Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2008; pp. 1032–1045. [Google Scholar]
- Green, J.R. Antitumor effects of bisphosphonates. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2003, 97, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Green, J.R. Bisphosphonates: Preclinical review. Oncologist 2004, 9, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Ravn, P.; Neugebauer, G.; Christiansen, C. Association between pharmacokinetics of oral ibandronate and clinical response in bone mass and bone turnover in women with postmenopausal osteoporosis. Bone 2002, 30, 320–324. [Google Scholar] [CrossRef]
- Hadji, P.; Coleman, R.E.; Wilson, C.; Powles, T.; Clézardin, P.; Aapro, M.; Costa, L.; Body, J.-J.; Markopoulos, C.; Santini, D. Adjuvant bisphosphonates in early breast cancer: Consensus guidance for clinical practice from a European Panel. Ann. Oncol. 2016, 27, 379–390. [Google Scholar] [CrossRef]
- Reyes, C.; Hitz, M.; Prieto-Alhambra, D.; Abrahamsen, B. Risks and benefits of bisphosphonate therapies. J. Cell. Biochem. 2016, 117, 20–28. [Google Scholar] [CrossRef]
- Lacerna, L.; Hohneker, J. Zoledronic acid for the treatment of bone metastases in patients with breast cancer and other solid tumors. Semin. Oncol. 2003, 30, 150–160. [Google Scholar] [CrossRef]
- Verron, E.; Bouler, J. Is bisphosphonate therapy compromised by the emergence of adverse bone disorders? Drug Discov. Today 2014, 198, 312–319. [Google Scholar] [CrossRef]
- Verron, E.; Khairoun, I.; Guicheux, J.; Bouler, J.-M. Calcium phosphate biomaterials as bone drug delivery systems: A review. Drug Discov. Today 2010, 15, 547–552. [Google Scholar] [CrossRef]
- Boanini, E.; Gazzano, M.; Bigi, A. Time course of zoledronate interaction with hydroxyapatite nanocrystals. J. Phys. Chem. C 2012, 116, 15812–15818. [Google Scholar] [CrossRef]
- Boanini, E.; Torricelli, P.; Gazzano, M.; Fini, M.; Bigi, A. The effect of zoledronate-hydroxyapatite nanocomposites on osteoclasts and osteoblast-like cells in vitro. Biomaterials 2012, 33, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Forte, L.; Torricelli, P.; Boanini, E.; Gazzano, M.; Fini, M.; Bigi, A. Antiresorptive and anti-angiogenetic octacalcium phosphate functionalized with bisphosphonates: An in vitro tri-culture study. Acta Biomater. 2017, 54, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.-B.; Darvell, B. Calcium phosphate solubility: The need for re-evaluation. Cryst. Growth Des. 2009, 9, 639–645. [Google Scholar] [CrossRef]
- Russell, R.; Watts, N.; Ebetino, F.; Rogers, M. Mechanisms of action of bisphosphonates: Similarities and differences and their potential influence on clinical efficacy. Osteoporos. Int. 2008, 19, 733–759. [Google Scholar] [CrossRef]
- Sato, M.; Grasser, W.; Endo, N.; Akins, R.; Simmons, H.; Thompson, D.; Golub, E.; Rodan, G. Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. J. Clin. Investig. 1991, 88, 2095–2105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nancollas, G.H.; Tang, R.; Phipps, R.J.; Henneman, Z.; Gulde, S.; Wu, W.; Mangood, A.; Russell, R.G.G.; Ebetino, F.H. Novel insights into actions of bisphosphonates on bone: Differences in interactions with hydroxyapatite. Bone 2006, 38, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Yu, T.; Ye, J. Microstructure and properties of alendronate-loaded calcium phosphate cement. Mater. Sci. Eng. C 2014, 42, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Garay, T.; Kenessey, I.; Molnár, E.; Juhász, É.; Réti, A.; László, V.; Rózsás, A.; Dobos, J.; Döme, B.; Berger, W. Prenylation inhibition-induced cell death in melanoma: Reduced sensitivity in BRAF mutant/PTEN wild-type melanoma cells. PLoS ONE 2015, 10, e0117021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tai, T.-W.; Chen, C.-Y.; Su, F.-C.; Tu, Y.-K.; Tsai, T.-T.; Lin, C.-F.; Jou, I.-M. Reactive oxygen species are required for zoledronic acid-induced apoptosis in osteoclast precursors and mature osteoclast-like cells. Sci. Rep. 2017, 7, 44245. [Google Scholar] [CrossRef]
- Junrui, P.; Bingyun, L.; Yanhui, G.; Xu, J.; Darko, G.M.; Dianjun, S. Relationship between fluoride exposure and osteoclast markers during RANKL-induced osteoclast differentiation. Environ. Toxicol. Pharmacol. 2016, 46, 241–245. [Google Scholar] [CrossRef]
- Zhou, H.; Chernecky, R.; Davies, J. Scanning electron microscopy of the osteoclast-bone interface in vivo. Cells Mater. 1993, 3, 2. [Google Scholar]
- Balvan, J.; Krizova, A.; Gumulec, J.; Raudenska, M.; Sladek, Z.; Sedlackova, M.; Babula, P.; Sztalmachova, M.; Kizek, R.; Chmelik, R. Multimodal holographic microscopy: Distinction between apoptosis and oncosis. PLoS ONE 2015, 10, e0121674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santini, D.; Schiavon, G.; Angeletti, S.; Vincenzi, B.; Gasparro, S.; Grilli, C.; La Cesa, A.; Virzi, V.; Leoni, V.; Budillon, A. Last generation of amino-bisphosphonates (N-BPs) and cancer angiogenesis: A new role for these drugs? Recent Pat. Anti-Cancer Drug Discov. 2006, 1, 383–396. [Google Scholar] [CrossRef]
- Chen, J.; Ashames, A.; Buabeid, M.A.; Fahelelbom, K.M.; Ijaz, M.; Murtaza, G. Nanocomposites drug delivery systems for the healing of bone fractures. Int. J. Pharm. 2020, 119477. [Google Scholar] [CrossRef]
- Eddy, A.; Tsuchiya, K.; Tsuru, K. Ishikawa, Fabrication of self-setting β-TCP granular cement using β-TCP granules and sodium hydrogen sulfate solution. J. Biomater. Appl. 2018, 33, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Mariño, F.T.; Torres, J.; Tresguerres, I.; Jerez, L.B.; Cabarcos, E.L. Vertical bone augmentation with granulated brushite cement set in glycolic acid. J. Biomed. Mater. Res. Part A 2007, 81, 93–102. [Google Scholar] [CrossRef]
- Kharazmi, M.; Persson, U.; Warfvinge, G. Pharmacovigilance of oral bisphosphonates: Adverse effects manifesting in the soft tissue of the oral cavity. J. Oral Maxillofac. Surg. 2012, 70, 2793–2797. [Google Scholar] [CrossRef]
- Lanza, F.L.; Hunt, R.H.; Thomson, A.B.; Provenza, J.M.; Blank, M.A.; Group, R.E.S. Endoscopic comparison of esophageal and gastroduodenal effects of risedronate and alendronate in postmenopausal women. Gastroenterology 2000, 119, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Ivanovski, S.; Slevin, M.; Hamlet, S.; Pop, T.S.; Brinzaniuc, K.; Petcu, E.B.; Miroiu, R.I. Bisphosphonate-related osteonecrosis of jaw (BRONJ): Diagnostic criteria and possible pathogenic mechanisms of an unexpected anti-angiogenic side effect. Vasc. Cell 2013, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Russell, R.G.G. Determinants of structure–function relationships among bisphosphonates. Bone 2007, 40, S21–S25. [Google Scholar] [CrossRef]
- Nishiguchi, A.; Taguchi, T. Osteoclast-responsive, injectable bone of bisphosphonated-nanocellulose that regulates osteoclast/osteoblast activity for bone regeneration. Biomacromolecules 2019, 20, 1385–1393. [Google Scholar] [CrossRef]
- Coxon, F.P.; Thompson, K.; Roelofs, A.J.; Ebetino, F.H.; Rogers, M.J. Visualizing mineral binding and uptake of bisphosphonate by osteoclasts and non-resorbing cells. Bone 2008, 42, 848–860. [Google Scholar] [CrossRef]
- Thompson, K.; Rogers, M.J.; Coxon, F.P.; Crockett, J.C. Cytosolic entry of bisphosphonate drugs requires acidification of vesicles after fluid-phase endocytosis. Mol. Pharmacol. 2006, 69, 1624–1632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merrell, M.A.; Wakchoure, S.; Ilvesaro, J.M.; Zinn, K.; Gehrs, B.; Lehenkari, P.P.; Harris, K.W.; Selander, K.S. Differential effects of Ca2+ on bisphosphonate-induced growth inhibition in breast cancer and mesothelioma cells. Eur. J. Pharmacol. 2007, 559, 21–31. [Google Scholar] [CrossRef]
- Huang, X.; Huang, S.; Guo, F.; Xu, F.; Cheng, P.; Ye, Y.; Dong, Y.; Xiang, W.; Chen, A. Dose-dependent inhibitory effects of zoledronic acid on osteoblast viability and function in vitro. Mol. Med. Rep. 2016, 13, 613–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scala, R.; Maqoud, F.; Angelelli, M.; Latorre, R.; Perrone, M.G.; Scilimati, A.; Tricarico, D. Zoledronic acid modulation of TRPV1 channel currents in osteoblast cell line and native rat and mouse bone marrow-derived osteoblasts: Cell proliferation and mineralization effect. Cancers 2019, 11, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.N.; Hwang, H.-S.; Oh, S.-H.; Roshanzadeh, A.; Kim, J.-W.; Song, J.H.; Kim, E.-S.; Koh, J.-T. Elevated extracellular calcium ions promote proliferation and migration of mesenchymal stem cells via increasing osteopontin expression. Exp. Mol. Med. 2018, 50, 1–16. [Google Scholar] [CrossRef]
- Misso, G.; Porru, M.; Stoppacciaro, A.; Castellano, M.; De Cicco, F.; Leonetti, C.; Santini, D.; Caraglia, M. Evaluation of the in vitro and in vivo antiangiogenic effects of denosumab and zoledronic acid. Cancer Biol. Ther. 2012, 13, 1491–1500. [Google Scholar] [CrossRef] [Green Version]
- Zafar, S.; Cullinan, M.P.; Drummond, B.K.; Seymour, G.J.; Coates, D.E. Effects of zoledronic acid and geranylgeraniol on angiogenic gene expression in primary human osteoclasts. J. Oral Sci. 2020, 62, 79–83. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.-Y.; Zheng, G.-S.; Wang, L.; Liang, Y.-J.; Zhang, S.-E.; Lao, X.-M.; Li, K.; Liao, G.-Q. Zoledronate suppressed angiogenesis and osteogenesis by inhibiting osteoclasts formation and secretion of PDGF-BB. PLoS ONE 2017, 12, e0179248. [Google Scholar] [CrossRef]
- Rahman, M.M.; Matsuoka, K.; Takeshita, S.; Ikeda, K. Secretion of PDGF isoforms during osteoclastogenesis and its modulation by anti-osteoclast drugs. Biochem. Biophys. Res. Commun. 2015, 462, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Kuljanin, J.; Janković, I.; Nedeljković, J.; Prstojević, D.; Marinković, V. Spectrophotometric determination of alendronate in pharmaceutical formulations via complex formation with Fe (III) ions. J. Pharm. Biomed. Anal. 2002, 28, 1215–1220. [Google Scholar] [CrossRef]











| Sample | Charged-ZOL | Loaded-ZOL | Zeta Potential | |
|---|---|---|---|---|
| (mmol/L) | (mass%) | pH 7.3 | pH 5.5 | |
| (mV) | (mV) | |||
| ZOL(0) | 0 | 0.00 | −9.46 ± 1.76 | −12.25 ± 2.41 |
| ZOL(1.5) | 1.5 | 1.07 ± 0.0422 | −10.44 ± 2.51 | −9.13 ± 1.49 |
| ZOL(3) | 3 | 2.11 ± 0.106 | −12.20 ± 1.50 | −6.19 ± 1.94 |
| ZOL(6) | 6 | 4.28 ± 0.0882 | −14.81 ± 1.77 | −0.96 ± 2.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kameda, Y.; Aizawa, M.; Sato, T.; Honda, M. Zoledronic Acid-Loaded β-TCP Inhibits Tumor Proliferation and Osteoclast Activation: Development of a Functional Bone Substitute for an Efficient Osteosarcoma Treatment. Int. J. Mol. Sci. 2021, 22, 1889. https://doi.org/10.3390/ijms22041889
Kameda Y, Aizawa M, Sato T, Honda M. Zoledronic Acid-Loaded β-TCP Inhibits Tumor Proliferation and Osteoclast Activation: Development of a Functional Bone Substitute for an Efficient Osteosarcoma Treatment. International Journal of Molecular Sciences. 2021; 22(4):1889. https://doi.org/10.3390/ijms22041889
Chicago/Turabian StyleKameda, Yuka, Mamoru Aizawa, Taira Sato, and Michiyo Honda. 2021. "Zoledronic Acid-Loaded β-TCP Inhibits Tumor Proliferation and Osteoclast Activation: Development of a Functional Bone Substitute for an Efficient Osteosarcoma Treatment" International Journal of Molecular Sciences 22, no. 4: 1889. https://doi.org/10.3390/ijms22041889