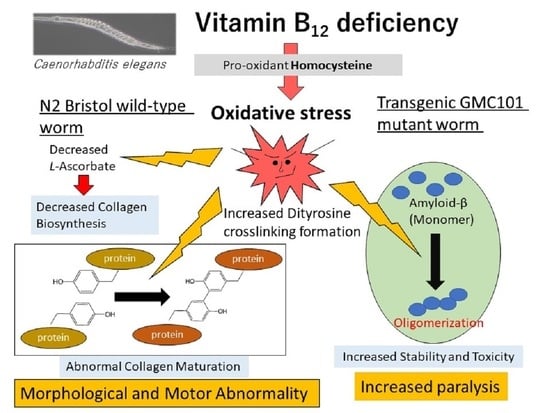
Dityrosine Crosslinking of Collagen and Amyloid-β Peptides Is Formed by Vitamin B12 Deficiency-Generated Oxidative Stress in Caenorhabditis elegans
Abstract
:1. Introduction
2. Results
2.1. Effect of B12 Deficiency on Collagen Biosynthesis in C. elegans
2.2. Effect of B12 Deficiency on the Dityrosine Crosslinking Level of Worm Collagen
2.3. Effect of Collagenase Treatment on the Cuticular Extracellular Matrix Epidermal Collagen Layer in Control and B12-Deficient Worms
2.4. Effect of B12 Deficiency on C. elegans Motility Function
2.5. Effect of B12 Deficiency on the Dityrosince Crosslinking Level of Aβ Peptides in GMC101 Worms
3. Discussion
4. Materials and Methods
4.1. Organisms
4.2. Worm Body Collagen Determination
4.3. AsA Determination
4.4. Dityrosine Determination
4.5. Collagenase Treatment
4.6. Assays of Malondialdehyde and H2O2 as Oxidative Stress Markers
4.7. Immunofluorescent Staining of Aβ and Dityrosine in GMC 101 Mutant Worms
4.8. Quantitative Polymerase Chain Reaction (qPCR) Analysis
4.9. Swim Locomotion Analysis
4.10. Paralysis Assay
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sokolovskaya, O.M.; Plessl, T.; Bailey, H.; Mackinnon, S.; Baumgartner, M.R.; Yue, W.W.; Froese, D.S.; Taga, M.E. Naturally occurring cobalamin (B12) analogs can function as cofactor for human methylmalonyl-CoA mutase. Biochimie 2021, 183, 35–43. [Google Scholar] [CrossRef]
- Froese, D.S.; Fowler, B.; Baumgartner, M.R. Vitamin B12, folate, and the methionine remethylation cycle—Biochemistry, pathways, and regulation. J. Inherit. Metab. Dis. 2018, 42, 673–685. [Google Scholar] [CrossRef] [Green Version]
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and Its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academies Press: Washington, DC, USA, 1998. [Google Scholar]
- Lu, S.C. S-Adenosylmethionine. Int. J. Biochem. Cell Biol. 2000, 32, 391–395. [Google Scholar] [CrossRef]
- Pepper, M.R.; Black, M.M. B12 in fetal development. Semin. Cell Dev. Biol. 2011, 22, 619–623. [Google Scholar] [CrossRef]
- Ebara, S.; Toyoshima, S.; Matsumura, T.; Adachi, S.; Takenaka, S.; Yamaji, R.; Watanabe, F.; Miyatake, K.; Inui, H.; Nakano, Y. Cobalamin deficiency results in severe metabolic disorder of serine and threonine in rats. Biochim. Biophys. Acta 2001, 1568, 111–117. [Google Scholar] [CrossRef]
- Bito, T.; Matsunaga, Y.; Yabuta, Y.; Kawano, T.; Watanabe, F. Vitamin B12 deficiency in Caenorhabditis elegans results in loss of fertility, extended life cycle, and reduced lifespan. FEBS Open Bio 2013, 3, 112–117. [Google Scholar] [CrossRef] [Green Version]
- Kavitha, O.; Thampan, R.V. Factors influencing collagen biosynthesis. J. Cell. Biochem. 2008, 104, 1150–1160. [Google Scholar] [CrossRef]
- Gorres, K.L.; Raines, R.T. Prolyl 4-hydroxylase. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 106–124. [Google Scholar] [CrossRef]
- Thaler, R.; Agsten, M.; Spitzer, S.; Paschalis, E.P.; Karlic, H.; Klaushofer, K.; Varga, F. Homocysteine suppresses the expression of the collagen cross-linker lysyl oxidase involving IL-6, Fli1, and Epigenetic DNA methylation. J. Biol. Chem. 2011, 286, 5578–5588. [Google Scholar] [CrossRef] [Green Version]
- Thein, M.C.; Winter, A.D.; Stepek, G.; McCormack, G.; Stapleton, G.; Johnstone, I.L.; Page, A.P. Combined extracellular matrix cross-linking activity of the peroxidase MLT-7 and the dual oxidase BLI-3 is critical for post-embryonic viability in Caenorhabditis elegans. J. Biol. Chem. 2009, 284, 17549–17563. [Google Scholar] [CrossRef] [Green Version]
- Ewald, C.Y. Redox signaling of NADPH oxidases regulates oxidative stress responses, immunity and aging. Antioxidants 2018, 7, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Hilaly, Y.K.; Williams, T.L.; Stewart-Parker, M.; Ford, L.; Skaria, E.; Cole, M.; Bucher, W.G.; Morris, K.L.; Sada, A.A.; Thorpe, J.R.; et al. A central role for dityrosine crosslinking of amyloid-β in Alzheimer’s disease. Acta Neuropathol. Commun. 2013, 1, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edens, W.A.; Sharling, L.; Cheng, G.; Shapira, R.; Kinkade, J.M.; Lee, T.; Edens, H.A.; Tang, X.; Sullards, C.; Flaherty, D.B.; et al. Tyrosine cross-linking of extracellular matrix is catalyzed by Duox, a multidomain oxidase/peroxidase with homology to the phagocyte oxidase subunit gp91phox. J. Cell Biol. 2001, 154, 879–892. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, S.; Hu, L.; Niu, H.; Sun, Q.; Li, W.; Tan, G.; Li, J.; Jin, L.; Lyu, J.; et al. Mitoferrin-1 is involved in the progression of Alzheimer’s disease through targeting mitochomdrial iron metablosm in a Caenorhabditis elegans model of Alzheimer’s disease. Neuroscience 2018, 385, 90–101. [Google Scholar] [CrossRef]
- Bito, T.; Misaki, T.; Yabuta, Y.; Ishikawa, T.; Kawano, T.; Watanabe, F. Vitamin B12 deficiency results in severe oxidative stress, leading to memory retention impairment in Caenorhabditis elegans. Redox Biol. 2017, 11, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Cai, L.; Phong, B.L.; Fisher, A.L.; Wang, Z. Regulation of fertility, survival, and cuticle collagen function by the Caenorhabditis elegans eaf-1 and ell-1 genes. J. Biol. Chem. 2011, 286, 35915–35921. [Google Scholar] [CrossRef] [Green Version]
- Page, A.P.; Stepek, G.; Winter, A.D.; Pertab, D. Enzymology of the nematode cuticle: A potential drug target? Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Kramer, J.M. Structures and functions of collagens in Caenorhabditis elegans. FASEB J. 1994, 8, 329–336. [Google Scholar] [CrossRef]
- Page, A.P.; Winter, A.D. Enzymes involved in the biogenesis of the nematode cuticle. Adv. Parasitol. 2003, 53, 85–148. [Google Scholar]
- Wormbook. The Cuticle. Available online: http://www.wormbook.org/index.html (accessed on 2 October 2021).
- Hill, A.A.; Hunter, C.P.; Tsung, B.T.; Tucker-Kellogg, G.; Brown, E.L. Genomic analysis of expression in C. elegans. Science 2000, 290, 809–812. [Google Scholar] [CrossRef] [Green Version]
- Chάvez, V.; Mohri-Shiomi, A.; Garsin, D.A. Ce-Duox1/BLI-3 generated reactive oxygen species as a protective innate immune mechanism in Caenorhabditis elegans. Infect. Immun. 2009, 77, 4983–4989. [Google Scholar] [CrossRef] [Green Version]
- Škovierová, H.; Vidomanová, E.; Mahmood, S.; Sopková, J.; Drgová, A.; Červeňová, T.; Halašová, E.; Lehotský, J. The molecular and cellular effect of homocysteine metabolism imbalance on human health. Int. J. Mol. Sci. 2016, 17, 1733. [Google Scholar] [CrossRef]
- Mahmoodian, F.; Peterkofsky, B. Vitamin C deficiency in guinea pigs differentially affects the expression of type 4 collagen, laminin, and elastin in blood vessels. J. Nutr. 1999, 129, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Clarke, M.; Ward, M.; Strain, J.J.; Hoey, L.; Dickey, W.; McNulty, H. B-vitamins and bone in health and disease: The current evidence. Proc. Nutr. Soc. 2014, 73, 330–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, M.; Marumo, K. The effects of homocysteine on the skeleton. Curr. Osteoporos. Rep. 2018, 16, 554–560. [Google Scholar] [CrossRef]
- Lévigne, D.; Modarressi, A.; Krause, K.H.; Pittet-Cuénod, B. NADPH oxidase 4 deficiency leads to impaired wound repair and reduced dityrosine-crosslinking, but does not affect myofibroblast formation. Free Radic. Biol. Med. 2016, 96, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, S.; Ji, L.; Wu, T.; Ma, F.; Ji, Y.; Zhou, Y.; Zheng, M.; Zhang, M.; Huang, G. Associations between Alzheimer’s disease and blood homocysteine, vitamin B12, and folate: A case-control study. Curr. Alzheimer Res. 2015, 12, 88–94. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.; Hong-Fang, J. Associations between homocysteine, folic acid, vitamin B12 and Alzheimer’s disease: Insights from meta-analyses. J. Alzheimer’s Dis. 2015, 46, 777–790. [Google Scholar]
- McLimans, K.E.; Martinez, A.D.C.; Mochel, J.P.; Allenspach, K. Serum vitamin B12 and related 5-methyltetrahydrofolate-homocysteine methyltransferase reductase and cubilin genotypes predict neural outcomes across the Alzheimer’s disease spectrum. Br. J. Nutr. 2020, 124, 135–145. [Google Scholar] [CrossRef] [Green Version]
- Sorrentino, V.; Romani, M.; Mouchiroud, L.; Beck, J.S.; Zhang, H.; D’Amico, D.; Moullan, N.; Potenza, F.; Schmid, A.W.; Rietsch, S.; et al. Enhancing mitochondrial proteostasis reduces amyloid-β proteotoxicity. Nature 2017, 552, 187–193. [Google Scholar] [CrossRef]
- Andra, A.; Tanigawa, S.; Bito, T.; Ishihara, A.; Watanabe, F.; Yabuta, Y. Effects of vitamin B12 deficiency on amyloid- toxicity in Caenorhabditis elegans. Antioxidants 2021, 10, 962. [Google Scholar] [CrossRef] [PubMed]
- Sitkiewicz, E.; Oledzki, J.; Poznański, J.; Dadlez, M. Di-tyrosine cross-link decreases the collisional cross-section of Aβ peptide dimers and trimers in the gas phase: An ion mobility study. PLoS ONE 2014, 9, e100200. [Google Scholar] [CrossRef] [PubMed]
- Maina, M.B.; Mengham, K.; Burra, G.K.; Al-Hilaly, Y.A.; Serpell, L.C. Dityrosine cross-link trapping of amyloid-β intermediates reveals that self-assembly is required for Aβ-induced cytotoxicity. bioRxiv 2020. [Google Scholar] [CrossRef]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Roach, D.; Gehrke, C.W. The hydrolysis of proteins. J. Chromatogr. A 1970, 52, 393–404. [Google Scholar] [CrossRef]
- Standard Tables of Food Composition in Japan-2010. The Council for Science and Technology, Ministry of Education, Culture, Sports, Science and Technolgy; Official Gazette Co-Operation of Japan: Tokyo, Japan, 2010.
- Gutierrez-Zepeda, A.; Santell, R.; Wu, Z.; Brown, M.; Wu, Y.; Khan, I.; Link, C.D.; Zhao, B.; Luo, Y. Soy isoflavone glycitein protects against beta amyloid-induced toxicity and oxidative stress in transgenic Caenorhabditis elegans. BMC Neurosci. 2005, 6, 54. [Google Scholar] [CrossRef] [Green Version]








| Genes | Sequence (5′–3′) |
|---|---|
| dpy-18 (Sense) | CTACCACACTGTGATGTGGATG |
| dpy-18 (Antisense) | GCGTGCTTCAAGTTGTTCTG |
| phy-2 (Sense) | GCTTGATGTGTGGATGCAGGTT |
| phy-2 (Antisense) | TTGCGAGTCGTTTGGTGAGA |
| pdi-2 (Sense) | CGGAATCGATGATGTTCCATTCGG |
| pdi-2 (Antisense) | TTGGGTGAGCTTCTCGTCGAAAG |
| bli-3 (Sense) | GCGCTCAAAACATGTGCTGT |
| bli-3 (Antisense) | GCCAGATTGTTGTACCATCCGT |
| mlt-7 (Sense) | TTGCGATCATCACGAGTGGTGT |
| mlt-7 (Antisense) | AGCAGTTGTCGTGACTGGCAAA |
| Aβ (Sense) | GCGGATGCAGAATTCCGACATGAC |
| Aβ (Antisense) | TATGACAACACCGCCCACCATGAG |
| sod-1 (Sense) | TCTTCTCACTCAGGTCTCCAAC |
| sod-1 (Antisense) | TCGGACTTCTGTGTGATCCA |
| ctl-1 (Sense) | ATTATGCTCGTGGTGGAAACCC |
| ctl-1 (Antisense) | ACAATGTTTGGCGCCCTCAA |
| act-1 (Sense) | TCCAAGAGAGAGGTATCCTTACCC |
| act-1 (Antisense) | CTCCATATCATCCCAGTTGGTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koseki, K.; Yamamoto, A.; Tanimoto, K.; Okamoto, N.; Teng, F.; Bito, T.; Yabuta, Y.; Kawano, T.; Watanabe, F. Dityrosine Crosslinking of Collagen and Amyloid-β Peptides Is Formed by Vitamin B12 Deficiency-Generated Oxidative Stress in Caenorhabditis elegans. Int. J. Mol. Sci. 2021, 22, 12959. https://doi.org/10.3390/ijms222312959
Koseki K, Yamamoto A, Tanimoto K, Okamoto N, Teng F, Bito T, Yabuta Y, Kawano T, Watanabe F. Dityrosine Crosslinking of Collagen and Amyloid-β Peptides Is Formed by Vitamin B12 Deficiency-Generated Oxidative Stress in Caenorhabditis elegans. International Journal of Molecular Sciences. 2021; 22(23):12959. https://doi.org/10.3390/ijms222312959
Chicago/Turabian StyleKoseki, Kyohei, Aoi Yamamoto, Keisuke Tanimoto, Naho Okamoto, Fei Teng, Tomohiro Bito, Yukinori Yabuta, Tsuyoshi Kawano, and Fumio Watanabe. 2021. "Dityrosine Crosslinking of Collagen and Amyloid-β Peptides Is Formed by Vitamin B12 Deficiency-Generated Oxidative Stress in Caenorhabditis elegans" International Journal of Molecular Sciences 22, no. 23: 12959. https://doi.org/10.3390/ijms222312959