Therapeutic Potential of Antimicrobial Peptides in Polymicrobial Biofilm-Associated Infections
Abstract
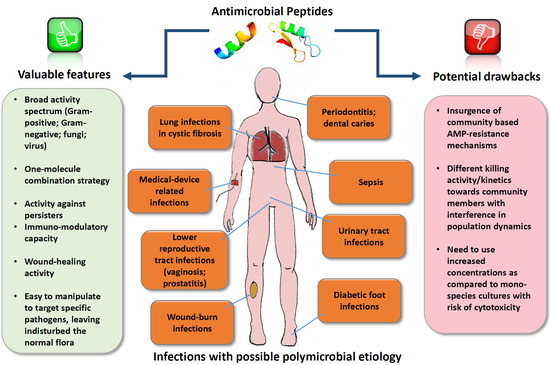
:1. Introduction
2. Bacteria–Bacteria Mixed Infections
2.1. Wound Infections
2.1.1. Conventional Therapy
2.1.2. AMP-Based Therapy
2.2. Respiratory Infections
2.2.1. Conventional Therapy
2.2.2. AMP-Based Therapy
2.3. Oral Infections
2.3.1. Conventional Therapies
2.3.2. AMP-Based Therapy
2.4. Sepsis
2.4.1. Conventional Therapies
2.4.2. AMP-Based Therapy
2.5. Infections of the Lower Female Reproductive Tract
2.5.1. Conventional Therapy
2.5.2. AMP-Based Therapy
3. Bacteria–Fungi Mixed Infections
3.1. Conventional Therapy
3.2. AMP-Based Therapy
4. Bacteria–Virus Mixed Infections
4.1. Conventional Therapy
4.2. AMP-Based Therapy
5. Single- or Multiple-Targeted AMPs to Discriminate Pathogens within Mixed Communities of Beneficial Bacteria
6. AMP Mimetics against Polymicrobial Infections
7. Potential Difficulties Arising in the Use of AMPs against Mixed Infections
8. Conclusions and Future Research Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Maisetta, G.; Batoni, G. Editorial: Interspecies interactions: Effects on virulence and antimicrobial susceptibility of bacterial and fungal pathogens. Front. Microbiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Filkins, L.M.; O’Toole, G.A. Cystic fibrosis lung infections: Polymicrobial, complex, and hard to treat. PLoS Pathog. 2015, 11, e1005258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limoli, D.H.; Hoffman, L.R. Help, hinder, hide and harm: What can we learn from the interactions between Pseudomonas aeruginosa and Staphylococcus aureus during respiratory infections? Thorax 2019, 74, 684–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanolkar, R.A.; Clark, S.T.; Wang, P.W.; Hwang, D.M.; Yau, Y.C.W.; Waters, V.J.; Guttman, D.S. Ecological succession of polymicrobial communities in the cystic fibrosis airways. mSystems 2020, 5, e00809-20. [Google Scholar] [CrossRef]
- Bertesteanu, S.; Triaridis, S.; Stankovic, M.; Lazar, V.; Chifiriuc, M.C.; Vlad, M.; Grigore, R. Polymicrobial wound infections: Pathophysiology and current therapeutic approaches. Int. J. Pharm. 2014, 463, 119–126. [Google Scholar] [CrossRef]
- Serra, R.; Grande, R.; Butrico, L.; Rossi, A.; Settimio, U.F.; Caroleo, B.; Amato, B.; Gallelli, L.; de Franciscis, S. Chronic wound infections: The role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert. Rev. Anti Infect. Ther. 2015, 13, 605–613. [Google Scholar] [CrossRef]
- Tomás, M.; Palmeira-de-Oliveira, A.; Simões, S.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R. Bacterial vaginosis: Standard treatments and alternative strategies. Int. J. Pharm. 2020, 587, 119659. [Google Scholar] [CrossRef]
- Javed, A.; Manzoor, S. Comparative analysis of bacterial vaginosis microbiota among pregnant and non-pregnant females and isolation of phages against Enterococcus faecalis, Enterococcus faecium, and Shigella flexneri strains. Microb. Pathog. 2020, 149, 104588. [Google Scholar] [CrossRef]
- Skerk, V.; Schönwald, S.; Krhen, I.; Markovinović, L.; Beus, A.; Kuzmanović, N.S.; Kruzić, V.; Vince, A. Aetiology of chronic prostatitis. Int. J. Antimicrob. Agents 2002, 19, 471–474. [Google Scholar] [CrossRef]
- Bair, K.L.; Campagnari, A.A. Moraxella catarrhalis promotes stable polymicrobial biofilms with the major otopathogens. Front. Microbiol. 2020, 10, 3006. [Google Scholar] [CrossRef] [Green Version]
- Azevedo, A.S.; Almeida, C.; Melo, L.F.; Azevedo, N.F. Impact of polymicrobial biofilms in catheter-associated urinary tract infections. Crit. Rev. Microbiol. 2017, 43, 423–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehrotra, N.; Singh, S. Periodontitis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar] [PubMed]
- Dahlen, G.; Basic, A.; Bylund, J. Importance of virulence factors for the persistence of oral bacteria in the inflamed gingival crevice and in the pathogenesis of periodontal disease. J. Clin. Med. 2019, 8, 1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sridhar, S.; Suprabha, B.S.; Shenoy, R.; Suman, E.; Rao, A. Association of Streptococcus mutans, Candida albicans and oral health practices with activity status of caries lesions among 5-year-old children with early childhood caries. Oral Health Prev. Dent. 2020, 18, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Kaya, E.; Tollapi, L.; Pastore, A.; Aringhieri, G.; Maisetta, G.; Barnini, S.; Paolicchi, A.; Batoni, G.; Esin, S. Comparison of methods for the microbiological diagnosis of totally implantable venous access port-related infections. J. Med. Microbiol. 2020. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms and device-associated infections. Emerg. Infect. Dis. 2001, 7, 277–281. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, A.; Andini, N.; Yang, S. A ‘culture’ shift: Application of molecular techniques for diagnosing polymicrobial infections. Biotechnol. Adv. 2019, 37, 476–490. [Google Scholar] [CrossRef]
- Weinstein, M.P.; Reller, L.B.; Murphy, J.R. Clinical importance of polymicrobial bacteremia. Diagn. Microbiol. Infect. Dis. 1986, 5, 185–196. [Google Scholar] [CrossRef]
- Klotz, S.A.; Chasin, B.S.; Powell, B.; Gaur, N.K.; Lipke, P.N. Polymicrobial bloodstream infections involving Candida species: Analysis of patients and review of the literature. Diagn. Microbiol. Infect. Dis. 2007, 59, 401–406. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Oglesby-Sherrouse, A.G. Interactions between Pseudomonas aeruginosa and Staphylococcus aureus during co-cultivations and polymicrobial infections. Appl. Microbiol. Biotechnol. 2016, 100, 6141–6148. [Google Scholar] [CrossRef]
- Dalton, T.; Dowd, S.E.; Wolcott, R.D.; Sun, Y.; Watters, C.; Griswold, J.A.; Rumbaugh, K.P. An in vivo polymicrobial biofilm wound infection model to study interspecies interactions. PLoS ONE 2011, 6, e27317. [Google Scholar] [CrossRef] [Green Version]
- DeLeon, S.; Clinton, A.; Fowler, H.; Everett, J.; Horswill, A.R.; Rumbaugh, K.P. Synergistic interactions of Pseudomonas aeruginosa and Staphylococcus aureus in an in vitro wound model. Infect. Immun. 2014, 82, 4718–4728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limoli, D.H.; Yang, J.; Khansaheb, M.K.; Helfman, B.; Peng, L.; Stecenko, A.A.; Goldberg, J.B. Staphylococcus aureus and Pseudomonas aeruginosa co-infection is associated with cystic fibrosis-related diabetes and poor clinical outcomes. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Hotterbeekx, A.; Kumar-Singh, S.; Goossens, H.; Malhotra-Kumar, S. In vivo and in vitro interactions between Pseudomonas aeruginosa and Staphylococcus spp. Front. Cell Infect. Microbiol. 2017, 7, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Medina, E.; Fan, D.; Coughlin, L.A.; Ho, E.X.; Lamont, I.L.; Reimmann, C.; Hooper, L.V.; Koh, A.Y. Candida albicans inhibits Pseudomonas aeruginosa virulence through suppression of pyochelin and pyoverdine biosynthesis. PLoS Pathog. 2015, 11, e1005129. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.P.; Fouhy, Y.; Garcia, B.F.; Watt, S.A.; Niehaus, K.; Yang, L.; Tolker-Nielsen, T.; Dow, J.M. Interspecies signalling via the Stenotrophomonas maltophilia diffusible signal factor influences biofilm formation and polymyxin tolerance in Pseudomonas aeruginosa. Mol. Microbiol. 2008, 68, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Harriott, M.M.; Noverr, M.C. Candida albicans and Staphylococcus aureus form polymicrobial biofilms: Effects on antimicrobial resistance. Antimicrob. Agents Chemother. 2009, 53, 3914–3922. [Google Scholar] [CrossRef] [Green Version]
- Orazi, G.; O’Toole, G.A. Pseudomonas aeruginosa alters Staphylococcus aureus sensitivity to vancomycin in a biofilm model of cystic fibrosis infection. mBio 2017, 8, e00873-17. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, L.R.; Deziel, E.; D’Argenio, D.A.; Lepine, F.; Emerson, J.; McNamara, S.; Gibson, R.L.; Ramsey, B.W.; Miller, S.I. Selection for Staphylococcus aureus small-colony variants due to growth in the presence of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2006, 103, 19890–19895. [Google Scholar] [CrossRef] [Green Version]
- Garcia, L.G.; Lemaire, S.; Kahl, B.C.; Becker, K.; Proctor, R.A.; Denis, O.; Tulkens, P.M.; Van Bambeke, F. Antibiotic activity against small-colony variants of Staphylococcus aureus: Review of in vitro, animal and clinical data. J. Antimicrob. Chemother. 2013, 68, 1455–1464. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Li, X.; Wang, Z.; Fu, Y.; Ai, Q.; Dong, Y.; Ju, J. Autoinducer-2 regulates Pseudomonas aeruginosa PAO1 biofilm formation and virulence production in a dose-dependent manner. BMC Microbiol. 2015, 15, 192. [Google Scholar] [CrossRef] [Green Version]
- Mashburn, L.M.; Jett, A.M.; Akins, D.R.; Whiteley, M. Staphylococcus aureus serves as an iron source for Pseudomonas aeruginosa during in vivo coculture. J. Bacteriol. 2005, 187, 554–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orazi, G.; O’Toole, G.A. “It takes a village”: Mechanisms underlying antimicrobial recalcitrance of polymicrobial biofilms. J. Bacteriol. 2019, 202, e00530-19. [Google Scholar] [CrossRef] [PubMed]
- Adam, B.; Baillie, G.S.; Douglas, L.J. Mixed species biofilms of Candida albicans and Staphylococcus epidermidis. J. Med. Microbiol. 2002, 51, 344–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nabb, D.L.; Song, S.; Kluthe, K.E.; Daubert, T.A.; Luedtke, B.E.; Nuxoll, A.S. Polymicrobial interactions induce multidrug tolerance in Staphylococcus aureus through energy depletion. Front. Microbiol. 2019, 10, 2803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lasa, I.; Solano, C. Polymicrobial infections: Do bacteria behave differently depending on their neighbors? Virulence 2018, 9, 895–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batoni, G.; Maisetta, G.; Esin, S. Antimicrobial peptides and their interaction with biofilms of medically relevant bacteria. Biochim. Biophys. Acta 2016, 1858, 1044–1060. [Google Scholar] [CrossRef]
- Riool, M.; de Breij, A.; Drijfhout, J.W.; Nibbering, P.H.; Zaat, S.A.J. Antimicrobial peptides in biomedical device manufacturing. Front Chem. 2017, 5, 63. [Google Scholar] [CrossRef]
- Di Luca, M.; Maccari, G.; Maisetta, G.; Batoni, G. BaAMPs: The database of biofilm-active antimicrobial peptides. Biofouling 2015, 31, 193–199. [Google Scholar] [CrossRef]
- Gupta, S.; Sharma, A.K.; Jaiswal, S.K.; Sharma, V.K. Prediction of biofilm inhibiting peptides: An in silico approach. Front. Microbiol. 2016, 7, 949. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, P.; Kumar, R.; Bhardwaj, A. DPABBs: A novel in silico approach for predicting and designing. Sci. Rep. 2016, 6, 21839. [Google Scholar] [CrossRef]
- Fallah Atanaki, F.; Behrouzi, S.; Ariaeenejad, S.; Boroomand, A.; Kavousi, K. BIPEP: Sequence-based prediction of biofilm inhibitory peptides using a combination of nmr and physicochemical descriptors. ACS Omega 2020, 5, 7290–7297. [Google Scholar] [CrossRef] [PubMed]
- Grassi, L.; Di Luca, M.; Maisetta, G.; Rinaldi, A.C.; Esin, S.; Trampuz, A.; Batoni, G. Generation of persister cells of Pseudomonas aeruginosa and Staphylococcus aureus by chemical treatment and evaluation of their susceptibility to membrane-targeting agents. Front. Microbiol. 2017, 8, 1917. [Google Scholar] [CrossRef] [PubMed]
- de Breij, A.; Riool, M.; Cordfunke, R.A.; Malanovic, N.; de Boer, L.; Koning, R.I.; Ravensbergen, E.; Franken, M.; van der Heijde, T.; Boekema, B.K.; et al. The antimicrobial peptide SAAP-148 combats drug-resistant bacteria and biofilms. Sci. Transl. Med. 2018, 10, eaan4044. [Google Scholar] [CrossRef] [Green Version]
- Mangoni, M.L.; McDermott, A.M.; Zasloff, M. Antimicrobial peptides and wound healing: Biological and therapeutic considerations. Exp. Dermatol. 2016, 25, 167–173. [Google Scholar] [CrossRef]
- Grassi, L.; Pompilio, A.; Kaya, E.; Rinaldi, A.C.; Sanjust, E.; Maisetta, G.; Crabbé, A.; Di Bonaventura, G.; Batoni, G.; Esin, S. The anti-microbial peptide (Lin-SB056-1)2-K reduces pro-inflammatory cytokine release through interaction with Pseudomonas aeruginosa lipopolysaccharide. Antibiotics 2020, 9, 585. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Fang, H.; Fang, L.; Liu, D.; Liu, J.; Su, M.; Fang, Z.; Ren, W.; Jiao, H. The Modification and design of antimicrobial peptide. Curr. Pharm. Des. 2018, 24, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jang, J.H.; Kim, S.C.; Cho, J.H. Development of a novel hybrid antimicrobial peptide for targeted killing of Pseudomonas aeruginosa. Eur. J. Med. Chem. 2020, 185, 111814. [Google Scholar] [CrossRef]
- Chung, E.M.C.; Dean, S.N.; Propst, C.N.; Bishop, B.M.; van Hoek, M.L. Komodo dragon-inspired synthetic peptide DRGN-1 promotes wound-healing of a mixed-biofilm infected wound. npj Biofilms Microbiomes 2017, 3, 9. [Google Scholar] [CrossRef]
- Gomes, D.; Santos, R.; Soares, R.S.; Reis, S.; Carvalho, S.; Rego, P.; Peleteiro, M.C.; Tavares, L.; Oliveira, M. Pexiganan in combination with nisin to control polymicrobial diabetic foot infections. Antibiotics 2020, 9, 128. [Google Scholar] [CrossRef] [Green Version]
- Jorge, P.; Grzywacz, D.; Kamysz, W.; LourencËo, A.; Pereira, M.O. Searching for new strategies against biofilm infections: Colistin-AMP combinations against Pseudomonas aeruginosa and Staphylococcus aureus single- and double-species biofilms. PLoS ONE 2017, 12, e0174654. [Google Scholar] [CrossRef] [Green Version]
- Bayramov, D.; Li, Z.; Patel, E.; Izadjoo, M.; Kim, H.; Neff, J. A novel peptide-based antimicrobial wound treatment is effective against biofilms of multi-drug resistant wound pathogens. Mil. Med. 2018, 183 (Suppl. 1), 481–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Z.; Wu, T.; Wang, W.; Li, B.; Wang, M.; Chen, L.; Xia, H.; Zhang, T. Biofunctions of antimicrobial peptide-conjugated alginate/hyaluronic acid/collagen wound dressings promote wound healing of a mixed-bacteria-infected wound. Int. J. Biol. Macromol. 2019, 140, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Ostorhazi, E.; Holub, M.C.; Rozgonyi, F.; Harmos, F.; Cassone, M.; Wade, J.D.; Otvos, L., Jr. Broad-spectrum antimicrobial efficacy of peptide A3-APO in mouse models of multidrug-resistant wound and lung infections cannot be explained by in vitro activity against the pathogens involved. Int. J. Antimicrob. Agents 2011, 37, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Gao, R.; Liu, C.; Shan, B.; Gao, F.; He, J.; Yuan, M.; Xie, H.; Jin, S.; Ma, Y. Potential role of the antimicrobial peptide Tachyplesin III against multidrug-resistant P. aeruginosa and A. baumannii coinfection in an animal model. Infect. Drug Resist. 2019, 12, 2865–2874. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.Y.; Cheng, J.W.; Yu, H.Y.; Lin, L.; Chih, Y.H.; Pan, Y.P. Efficacy of a novel antimicrobial peptide against periodontal pathogens in both planktonic and polymicrobial biofilm states. Acta Biomater. 2015, 25, 150–161. [Google Scholar] [CrossRef]
- Su, B.C.; Huang, H.N.; Lin, T.W.; Hsiao, C.D.; Chen, J.Y. Epinecidin-1 protects mice from LPS-induced endotoxemia and cecal ligation and puncture-induced polymicrobial sepsis. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 3028–3037. [Google Scholar] [CrossRef]
- Schuerholz, T.; Doemming, S.; Hornef, M.; Martin, L.; Simon, T.P.; Heinbockel, L.; Brandenburg, K.; Marx, G. The anti-inflammatory effect of the synthetic antimicrobial peptide 19-2.5 in a murine sepsis model: A prospective randomized study. Crit. Care 2013, 17, R3. [Google Scholar] [CrossRef] [Green Version]
- Martin, L.; Horst, K.; Chiazza, F.; Oggero, S.; Collino, M.; Brandenburg, K.; Hildebrand, F.; Marx, G.; Thiemermann, C.; Schuerholz, T. The synthetic antimicrobial peptide 19-2.5 attenuates septic cardiomyopathy and prevents down-regulation of SERCA2 in polymicrobial sepsis. Sci. Rep. 2016, 6, 37277. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, Y.; Chen, M.; Hu, C.; Chen, Y. Functional synergy of antimicrobial peptides and chlorhexidine acetate against Gram-negative/Gram-positive bacteria and a fungus in vitro and in vivo. Infect. Drug Resist. 2019, 12, 3227–3239. [Google Scholar] [CrossRef] [Green Version]
- de Alteriis, E.; Lombardi, L.; Falanga, A.; Napolano, M.; Galdiero, S.; Siciliano, A.; Carotenuto, R.; Guida, M.; Galdiero, E. Polymicrobial antibiofilm activity of the membranotropic peptide gH625 and its analogue. Microb. Pathog. 2018, 125, 189–195. [Google Scholar] [CrossRef]
- Gupta, S.; Thakur, J.; Pal, S.; Gupta, R.; Mishra, D.; Kumar, S.; Yadav, K.; Saini, A.; Yavvari, P.S.; Vedantham, M.; et al. Cholic acid-peptide conjugates as potent antimicrobials against interkingdom polymicrobial biofilms. Antimicrob. Agents Chemother. 2019, 63, e00520-19. [Google Scholar] [CrossRef] [PubMed]
- Melvin, J.A.; Lashua, L.P.; Kiedrowski, M.R.; Yang, G.; Deslouches, B.; Montelaro, R.C.; Bomberger, J.M. Simultaneous antibiofilm and antiviral activities of an engineered antimicrobial peptide during virus-bacterium coinfection. mSphere 2016, 1, e00083-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rollins-Smith, L.A.; Smith, P.B.; Ledeczi, A.M.; Rowe, J.M.; Reinert, L.K. Caerin 1 antimicrobial peptides that inhibit HIV and Neisseria may spare protective Lactobacilli. Antibiotics 2020, 9, 661. [Google Scholar] [CrossRef] [PubMed]
- Bessa, L.J.; Manickchand, J.R.; Eaton, P.; Leite, J.R.S.A.; Brand, G.D.; Gameiro, P. Intragenic antimicrobial peptide Hs02 hampers the proliferation of single- and dual-species biofilms of P. aeruginosa and S. aureus: A promising agent for mitigation of biofilm-associated infections. Int. J. Mol. Sci. 2019, 20, 3604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, Y.; Locock, K.; Verma-Gaur, J.; Hay, I.D.; Meagher, L.; Traven, A. Searching for new strategies against polymicrobial biofilm infections: Guanylated polymethacrylates kill mixed fungal/bacterial biofilms. J. Antimicrob. Chemother. 2016, 71, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Bolt, H.L.; Eggimann, G.A.; McAuley, D.F.; McMullan, R.; Curran, T.; Zhou, M.; Jahoda, P.C.; Cobb, S.L.; Lundy, F.T. Peptoid efficacy against polymicrobial biofilms determined by using propidium monoazide-modified quantitative PCR. ChemBioChem 2017, 18, 111–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Järbrink, K.; Ni, G.; Sönnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. Prevalence and incidence of chronic wounds and related complications: A protocol for a systematic review. Syst. Rev. 2016, 5, 152. [Google Scholar] [CrossRef] [Green Version]
- Metcalf, D.G.; Bowler, P.G. Biofilm delays wound healing: A review of the evidence. Burns Trauma 2013, 1, 5–12. [Google Scholar] [CrossRef] [Green Version]
- Harding, K. Innovation and wound healing. J. Wound Care 2015, 24, 7–13. [Google Scholar] [CrossRef]
- Wilson, J.R.; Mills, J.G.; Prather, I.D. A toxicity index of skin and wound cleansers used on in vitro fibroblasts and keratinocytes. Adv. SkinWound Care 2005, 18, 373–378. [Google Scholar] [CrossRef]
- Zhang, X.; Shu, W.; Yu, Q.; Qu, W.; Wang, Y.; Li, R. Functional biomaterials for treatment of chronic wound. Front. Bioeng. Biotechnol. 2020, 8, 516. [Google Scholar] [CrossRef] [PubMed]
- Kwiecien, K.; Zegar, A.; Jung, J.; Brzoza, P.; Kwitniewski, M.; Godlewska, U.; Grygier, B.; Kwiecinska, P.; Morytko, A.; Cichy, J. Architecture of antimicrobial skin defense. Cytokine Growth Factor Rev. 2019, 49, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Gallo, R.L. Antimicrobial peptides. Curr. Biol. 2016, 26, R14–R19. [Google Scholar] [CrossRef] [PubMed]
- Thapa, R.K.; Diep, D.B.; Tønnesen, H.H. Topical antimicrobial peptide formulations for wound healing: Current developments and future prospects. Acta Biomater. 2020, 103, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Woodburn, K.W.; Jaynes, J.M.; Clemens, L.E. Evaluation of the antimicrobial peptide, RP557, for the broad-spectrum treatment of wound pathogens and biofilm. Front. Microbiol. 2019, 10, 1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duplantier, A.J.; van Hoek, M.L. The human cathelicidin antimicrobial peptide LL-37 as a potential treatment for polymicrobial infected wounds. Front. Immunol. 2013, 4, 143. [Google Scholar] [CrossRef] [Green Version]
- Grassi, L.; Maisetta, G.; Esin, S.; Batoni, G. Combination strategies to enhance the efficacy of antimicrobial peptides against bacterial biofilms. Front. Microbiol. 2017, 8, 2409. [Google Scholar] [CrossRef]
- Christ, K.; Wiedemann, I.; Bakowsky, U.; Sahl, H.-G.; Bendas, G. The role of lipid II in membrane binding of and pore formation by nisin analyzed by two combined biosensor techniques. Biochim. Biophys. Acta Biomembr. 2007, 1768, 694–704. [Google Scholar] [CrossRef] [Green Version]
- Sandreschi, S.; Piras, A.M.; Batoni, G.; Chiellini, F. Perspectives on polymeric nanostructures for the therapeutic application of antimicrobial peptides. Nanomedicine 2016, 11, 1729–1744. [Google Scholar] [CrossRef] [Green Version]
- Piras, A.M.; Maisetta, G.; Sandreschi, S.; Gazzarri, M.; Bartoli, C.; Grassi, L.; Esin, S.; Chiellini, F.; Batoni, G. Chitosan nanoparticles loaded with the antimicrobial peptide temporin B exert a long-term antibacterial activity in vitro against clinical isolates of Staphylococcus epidermidis. Front. Microbiol. 2015, 6, 372. [Google Scholar] [CrossRef] [Green Version]
- Burns, J.L.; Rolain, J.M. Culture-based diagnostic microbiology in cystic fibrosis: Can we simplify the complexity? J. Cyst Fibros. 2014, 13, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiemstra, P.S. The role of epithelial beta-defensins and cathelicidins in host defense of the lung. Exp. Lung Res. 2007, 33, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Zhang, N.; Yang, J.; Meng, X.; Yang, R.; Li, J.; Sun, T. Antimicrobial peptide LL-37 and IDR-1 ameliorate MRSA pneumonia in vivo. Cell Physiol. Biochem. 2013, 32, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Barlow, P.G.; Svoboda, P.; Mackellar, A.; Nash, A.A.; York, I.A.; Pohl, J.; Davidson, D.J.; Donis, R.O. Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37. PLoS ONE 2011, 6, e25333. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, P.E.; McHugh, B.; Gwyer Findlay, E.; Mackellar, A.; Mackenzie, K.J.; Gallo, R.L.; Govan, J.R.; Simpson, A.J.; Davidson, D.J. Cathelicidin host defence peptide augments clearance of pulmonary Pseudomonas aeruginosa infection by its influence on neutrophil function in vivo. PLoS ONE 2014, 9, e99029. [Google Scholar] [CrossRef]
- Sczepanik, F.S.C.; Grossi, M.L.; Casati, M.; Goldberg, M.; Glogauer, M.; Fine, N.; Tenenbaum, H.C. Periodontitis is an inflammatory disease of oxidative stress: We should treat it that way. Periodontol 2000 2020, 84, 45–68. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Matesanz, P.; Bascones-Martínez, A.; Sanz, M. Local and systemic antimicrobial therapy in periodontics. J. Evid. Based Dent. Pract. 2012, 12 (Suppl. 3), 50–60. [Google Scholar] [CrossRef]
- Ardila, C.M.; Bedoya-García, J.A. Antimicrobial resistance of Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis and Tannerella forsythia in periodontitis patients. J. Glob. Antimicrob. Resist. 2020, 22, 215–218. [Google Scholar] [CrossRef]
- Dale, B.A.; Fredericks, L.P. Antimicrobial peptides in the oral environment: Expression and function in health and disease. Curr. Issues Mol. Biol. 2005, 7, 119–133. [Google Scholar] [CrossRef]
- Brancatisano, F.L.; Maisetta, G.; Barsotti, F.; Esin, S.; Miceli, M.; Gabriele, M.; Giuca, M.R.; Campa, M.; Batoni, G. Reduced human beta defensin 3 in individuals with periodontal disease. J. Dent. Res. 2011, 90, 241–245. [Google Scholar] [CrossRef]
- Maisetta, G.; Batoni, G.; Esin, S.; Luperini, F.; Pardini, M.; Bottai, D.; Florio, W.; Giuca, M.R.; Gabriele, M.; Campa, M. Activity of human beta-defensin 3 alone or combined with other antimicrobial agents against oral bacteria. Antimicrob. Agents Chemother. 2003, 47, 3349–3351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maisetta, G.; Batoni, G.; Esin, S.; Raco, G.; Bottai, D.; Favilli, F.; Florio, W.; Campa, M. Susceptibility of Streptococcus mutans and Actinobacillus actinomycetemcomitans to bactericidal activity of human beta-defensin 3 in biological fluids. Antimicrob. Agents Chemother. 2005, 49, 1245–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khurshid, Z.; Zafar, M.S.; Naseem, M.; Khan, R.S.; Najeeb, S. Human oral defensins antimicrobial peptides: A future promising antimicrobial drug. Curr. Pharm. Des. 2018, 24, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.Y.; Tu, C.H.; Yip, B.S.; Chen, H.L.; Cheng, H.T.; Huang, K.C.; Lo, H.J.; Cheng, J.W. Easy strategy to increase salt resistance of antimicrobial peptides. Antimicrob. Agents Chemother. 2011, 55, 4918–4921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.H.; Jun, H.K.; Lee, H.R.; Chung, C.P.; Choi, B.K. Antibacterial and lipopolysaccharide (LPS)-neutralising activity of human cationic antimicrobial peptides against periodontopathogens. Int. J. Antimicrob. Agents 2010, 35, 138–145. [Google Scholar] [CrossRef]
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K. International forum of acute care trialists. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef]
- Crnich, C.J.; Maki, D.G. The role of intravascular devices in sepsis. Curr. Infect. Dis. Rep. 2001, 3, 496–506. [Google Scholar] [CrossRef]
- Bustos, C.; Aguinaga, A.; Carmona-Torre, F.; Del Pozo, J.L. Long-term catheterization: Current approaches in the diagnosis and treatment of port-related infections. Infect Drug Resist. 2014, 7, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Zheng, C.; Zhang, S.; Chen, Q.; Zhong, L.; Huang, T.; Zhang, X.; Zhang, K.; Zhou, H.; Cai, J.; Du, L.; et al. Clinical characteristics and risk factors of polymicrobial Staphylococcus aureus bloodstream infections. Antimicrob. Resist. Infect. Control 2020, 9, 76. [Google Scholar] [CrossRef]
- Dejager, L.; Pinheiro, I.; Dejonckheere, E.; Libert, C. Cecal ligation and puncture: The gold standard model for polymicrobial sepsis? Trends Microbiol. 2011, 19, 198–208. [Google Scholar] [CrossRef]
- Tidbury, F.; Langhart, A.; Weidlinger, S.; Stute, P. Non-antibiotic treatment of bacterial vaginosis-a systematic review. Arch. Gynecol. Obstet. 2020. [Google Scholar] [CrossRef] [PubMed]
- Allison, D.L.; Willems, H.M.E.; Jayatilake, J.A.M.S.; Bruno, V.M.; Peters, B.M.; Shirtliff, M.E. Candida-bacteria interactions: Their impact on human disease. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Peleg, A.Y.; Hogan, D.A.; Mylonakis, E. Medically important bacterial–fungal interactions. Nat. Rev. Microbiol. 2010, 8, 340–349. [Google Scholar] [CrossRef]
- Neely, A.N.; Law, E.J.; Holder, I.A. Increased susceptibility to lethal Candida infections in burned mice preinfected with Pseudomonas aeruginosa or pretreated with proteolytic enzymes. Infect. Immun. 1986, 52, 200–204. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Jang, J.H.; Kim, S.C.; Cho, J.H. De novo generation of short antimicrobial peptides with enhanced stability and cell specificity. J. Antimicrob. Chemother. 2014, 69, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, L.; Maisetta, G.; Batoni, G.; Tavanti, A. Insights into the antimicrobial properties of hepcidins: Advantages and drawbacks as potential therapeutic agents. Molecules 2015, 20, 6319–6341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.; McLean, D.T.; Linden, G.J.; McAuley, D.F.; McMullan, R.; Lundy, F.T. The naturally occurring host defense peptide, LL-37, and its truncated mimetics KE-18 and KR-12 have selected biocidal and antibiofilm activities against Candida albicans, Staphylococcus aureus, and Escherichia coli in vitro. Front. Microbiol. 2017, 8, 544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falanga, A.; Vitiello, M.T.; Cantisani, M.; Tarallo, R.; Guarnieri, D.; Mignogna, E.; Netti, P.; Pedone, C.; Galdiero, M.; Galdiero, S. A peptide derived from herpes simplex virus type 1 glycoprotein H: Membrane translocation and applications to the delivery of quantum dots. Nanomedicine 2011, 7, 925–934. [Google Scholar] [CrossRef]
- Neu, U.; Mainou, B.A. Virus interactions with bacteria: Partners in the infectious dance. PLoS Pathog. 2020, 16, e1008234. [Google Scholar] [CrossRef]
- van Ewijk, B.E.; van der Zalm, M.M.; Wolfs, T.F.; van der Ent, C.K. Viral respiratory infections in cystic fibrosis. J. Cyst. Fibros. 2005, 4 (Suppl. 2), 31–36. [Google Scholar] [CrossRef] [Green Version]
- Hendricks, M.R.; Lashua, L.P.; Fischer, D.K.; Flitter, B.A.; Eichinger, K.M.; Durbin, J.E.; Sarkar, S.N.; Coyne, C.B.; Empey, K.M.; Bomberger, J.M. Respiratory syncytial virus infection enhances Pseudomonas aeruginosa biofilm growth through dysregulation of nutritional immunity. Proc. Natl. Acad. Sci. USA 2016, 113, 1642–1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tossi, A.; Sandri, L.; Giangaspero, A. Amphipathic, alpha-helical antimicrobial peptides. Biopolymers 2000, 55, 4–30. [Google Scholar] [CrossRef]
- Muhle, S.A.; Tam, J.P. Design of Gram-negative selective antimicrobial peptides. Biochemistry 2001, 40, 5777–5785. [Google Scholar] [CrossRef] [PubMed]
- Batoni, G.; Casu, M.; Giuliani, A.; Luca, V.; Maisetta, G.; Mangoni, M.L.; Manzo, G.; Pintus, M.; Pirri, G.; Rinaldi, A.C.; et al. Rational modification of a dendrimeric peptide with antimicrobial activity: Consequences on membrane-binding and biological properties. Amino Acids 2016, 48, 887–900. [Google Scholar] [CrossRef]
- Manzo, G.; Ferguson, P.M.; Gustilo, V.B.; Hind, C.K.; Clifford, M.; Bui, T.T.; Drake, A.F.; Atkinson, R.A.; Sutton, J.M.; Batoni, G.; et al. Minor sequence modifications in temporin B cause drastic changes in antibacterial potency and selectivity by fundamentally altering membrane activity. Sci. Rep. 2019, 9, 1385. [Google Scholar] [CrossRef] [Green Version]
- Eckert, R.; He, J.; Yarbrough, D.K.; Qi, F.; Anderson, M.H.; Shi, W. Targeted killing of Streptococcus mutans by a pheromone-guided “Smart” antimicrobial peptide. Antimicrob. Agents Chemother. 2006, 50, 3651–3657. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, R.; Santarpia, P.; Lavender, S.; Gittins, E.; Liu, Z.; Anderson, M.H.; He, J.; Shi, W.; Eckert, R. Clinical efficacy of a specifically targeted antimicrobial peptide mouth rinse: Targeted elimination of Streptococcus mutans and prevention of demineralization. Caries Res. 2011, 45, 415–428. [Google Scholar] [CrossRef] [Green Version]
- Eckert, R.; Qi, F.; Yarbrough, D.K.; He, J.; Anderson, M.H.; Shi, W. Adding selectivity to antimicrobial peptides: Rational design of a multidomain peptide against Pseudomonas spp. Antimicrob. Agents Chemother. 2006, 50, 1480–1488. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Anderson, M.H.; Shi, W.; Eckert, R. Design and activity of a ‘dual-targeted’ antimicrobial peptide. Int. J. Antimicrob. Agents 2009, 33, 532–537. [Google Scholar] [CrossRef] [Green Version]
- Zuckermann, R.N.; Kodadek, T. Peptoids as potential therapeutics. Curr. Opin. Mol. Ther. 2009, 11, 299–307. [Google Scholar]
- Miller, S.M.; Simon, R.J.; Ng, S.; Zuckermann, R.N.; kerr, J.M.; Moos, W.H. Comparison of the proteolytic susceptibilities of homologous L-Amino Acid, D-Amino Acid, and N-Substituted glycine peptide and peptoid oligomers. Drug Develop. Res. 1995, 35, 20–32. [Google Scholar] [CrossRef]
- Pompilio, A.; Crocetta, V.; De Nicola, S.; Verginelli, F.; Fiscarelli, E.; Di Bonaventura, G. Cooperative pathogenicity in cystic fibrosis: Stenotrophomonas maltophilia modulates Pseudomonas aeruginosa virulence in mixed biofilm. Front. Microbiol. 2015, 6, 951. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, S.; Limoli, D.H.; English, A.E.; Parsek, M.R.; Wozniak, D.J. Mixed communities of mucoid and non-mucoid Pseudomonas aeruginosa exhibit enhanced resistance to host antimicrobials. mBio 2018, 9, e00275-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Billings, N.; Millan, M.; Caldara, M.; Rusconi, R.; Tarasova, Y.; Stocker, R.; Ribbeck, K. The extracellular matrix component Psl provides fast-acting antibiotic defense in Pseudomonas aeruginosa biofilms. PLoS Pathog. 2013, 9, e1003526. [Google Scholar] [CrossRef] [Green Version]
- Gläser, R.; Becker, K.; von Eiff, C.; Meyer-Hoffert, U.; Harder, J. Decreased susceptibility of Staphylococcus aureus small-colony variants toward human antimicrobial peptides. J. Investig. Dermatol. 2014, 134, 2347–2350. [Google Scholar] [CrossRef] [Green Version]
- Steadman, R.; Heck, L.W.; Abrahamson, D.R. The role of proteases in the pathogenesis of Pseudomonas aeruginosa infections. In Pseudomonas aeruginosa as an Opportunistic Pathogen. Infectious Agents and Pathogenesis; Campa, M., Bendinelli, M., Friedman, H., Eds.; Springer: Boston, MA, USA, 1993. [Google Scholar] [CrossRef]
- Kuramitsu, H.K. Proteases of Porphyromonas gingivalis: What don’t they do? Oral Microbiol. Immunol. 1998, 13, 263–270. [Google Scholar] [CrossRef]
- Joo, H.S.; Fu, C.I.; Otto, M. Bacterial strategies of resistance to antimicrobial peptides. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150292. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.J.; Park, S.J.; Mishig-Ochir, T.; Lee, B.J. Antimicrobial peptides: Therapeutic potentials. Expert Rev. Anti Infect. Ther. 2014, 12, 1477–1486. [Google Scholar] [CrossRef]
- Rončević, T.; Puizina, J.; Tossi, A. Antimicrobial peptides as anti-infective agents in pre-post-antibiotic era? Int. J. Mol. Sci. 2019, 20, 5713. [Google Scholar] [CrossRef] [Green Version]
- Browne, K.; Chakraborty, S.; Chen, R.; Willcox, M.D.; Black, D.S.; Walsh, W.R.; Kumar, N. A new era of antibiotics: The clinical potential of antimicrobial peptides. Int. J. Mol. Sci. 2020, 21, 7047. [Google Scholar] [CrossRef]



| Types of Infections with Possible Polymicrobial Etiology | Common Species Involved | References |
|---|---|---|
| Lung infections in cystic fibrosis | Pseudomonas aeruginosa, Staphylococcus aureus, Haemophilus influenzae, Burkholderia cepacia complex, Candida albicans, respiratory syncytial virus | [2,3,4] |
| Chronic wounds (wound burn infections, diabetic wound infections) | S. aureus, coagulase-negative staphylococci, P. aeruginosa, Escherichia coli, Klebsiella spp., Enterobacter spp., Enterococcus spp. beta-hemolytic streptococci, Candida spp. | [5,6] |
| Vaginosis | Gardnerella vaginalis, Atopobium vaginae, Peptostreptococci, Prevotella spp., Mobiluncus spp., Mycoplasma spp., Ureaplasma urealyticum, Fusobacterium nucleatum, E. faecalis | [7,8] |
| Prostatitis | Chlamydia trachomatis, U. urealyticum, Mycoplasma hominis, Trichomonas vaginalis, E. coli, Enterococci | [9] |
| Otitis media | Streptococcus pneumoniae, H. influenzae, Moraxella catarrhalis | [10] |
| Urinary tract infections | E. coli, Proteus mirabilis, E. faecalis, K. pneumoniae, P. aeruginosa | [11] |
| Periodontitis | Porphyromonas gingivalis, Tannerella forsythia, Treponema denticola | [12,13] |
| Dental caries | S. mutans, C. albicans | [14] |
| Medical device-related infections | Coagulase-negative Staphylococci, S. aureus, E. faecalis, P. aeruginosa, C. albicans, K. pneumoniae | [15,16] |
| Sepsis following dissemination | Enterobacteriaceae, non-group A streptococci, anaerobic bacteria, Staphylococci, Pseudomonas spp. Candida spp. | [17,18,19] |
| Property | AMPs | Conventional Antibiotics |
|---|---|---|
| Activity spectrum | Generally broad (directed against Gram-positive, Gram-negative, fungi and virus), and possibly able to accomplish a one-molecule combination strategy | Generally narrow, especially last resort antibiotics |
| Anti-persister activity | Demonstrated for many AMPs | None or poor |
| Immuno-modulatory capacity | Demonstrated for many AMPs | None or poor |
| Wound-healing activity | Demonstrated for many AMPs | None or poor |
| Prone to manipulations | Easy to manipulate to improve antimicrobial activity/reduce toxicity | Difficult to manipulate |
| Activity against beneficial flora | Possibly able to target AMPs against specific pathogens, leaving undisturbed the normal flora | Active against beneficial flora |
| Induction of resistance | Generally low; in some cases induction of resistance after several passages in vitro. In the case of polymicrobial infections, possibility of insurgence of community-based AMP-resistance mechanisms | Resistance easily induced. In the case of polymicrobial infections, interspecies interactions may affect the antibiotic susceptibility of individual organisms. |
| Stability in biological fluids | Generally low unless modifications are made | Generally high |
| Active concentrations | The need to use increased concentrations as compared to mono-species biofilms has been reported with a consequent risk of cytotoxicity | Therapeutic concentrations against susceptible strains highly optimized |
| Approval by drug agencies | Difficult; only very few AMPs approved for clinical use | Approval of new antibiotics is slower than needed. Only few large pharmaceutical companies have ongoing antibiotic discovery programs |
| AMPs | Sequence a or Molecular Formula | Co-Infecting Species | Type of Application/Infection Model | Ref. |
|---|---|---|---|---|
| DRGN-1 | PSKKTKPVKPKKVA | P. aeruginosa and S. aureus | In vitro co-infection model and mouse model of wound infection | [49] |
| Pexiganan-nisin (dual-AMP) | GIGKFLKKAKKFGKAFVKILKK-NH2 C143H230N42O37S7 | S. aureus and P. aeruginosa | Dual AMP biogel/collagen three-dimensional (3D) model | [50] |
| CST sulfate salt TP-I-L CIT-1.1 TEMP-A | C53H102N16O17S KWCFRVCYRGICYRRCR-NH2 GLFDVIKKVASVIGGL-NH2 FLPLIGRVLSGIL-NH2 | S. aureus and P. aeruginosa | In vitro co-infection model | [51] |
| ASP-1 | RRWVRRVRRWVRRVVRVVRRWVRR | S. aureus, A. baumannii, K. pneumoniae, and P. aeruginosa | hydrophilic polyurethane (PU)-based dressing/in vitro co-infection model | [52] |
| Tet213 | KRWWKWWRRC | E. coli and S. aureus | Peptide-immobilized ALG/HA/COL wound dressings and rat model of wound infection | [53] |
| A3-APO | [(1-amino-cyclohexane carboxylic acid-RPDKPRPYLPRPRPPRPVR)2-2,4-diamino-butyric acid]-NH2 | K. pneumoniae, A. baumannii, and P. mirabilis | mouse model of wound infection | [54] |
| Tachyplesin III | KWCFRVCYRGICYRKCR-NH2 | P. aeruginosa and A. baumannii | Mouse model of bacterial co-infection pneumonia | [55] |
| Nal-P-113 | AKR-Nal-Nal-GYKRKF-Nal-NH2 | F. nucleatum, S. gordonii, and P. gingivalis | In vitro artificial saliva-coated hydroxyapatite co-infection model | [56] |
| Epinecidin-1 | GFIFHIIKGLFHAGKMIHGLV | Gut microflora | Mouse model of polymicrobial sepsis and LPS-induced endotoxemia | [57] |
| Pep19-2.5 | GCKKYRRFRWKFKGKFWFWG-NH2 | Gut microflora | Mouse model of polymicrobial sepsis | [58,59] |
| HPRP-A2 | Nα-acetyl-FKKLKKLFSKLWNWK-NH2 | E. coli and S. aureus | Rat bacterial vaginitis | [60] |
| gH625 gH625-GCGKKKK | HGLASTLTRWAHYNALIRAF HGLASTLTRWAHYNALIRAF-GCGKKKK | C. tropicalis and S. marcescens or C. tropicalis and S. aureus | In vitro co-infection model | [61] |
| CAP-3 | CA-V3 | S. aureus and C. albicans | In vitro co-infection model. Murine wound and catheter infection models | [62] |
| WLBU2 | RRWVRRVRRWVRRVVRVVRRWVRR | P. aeruginosa and Respiratory syncytial virus | In vitro co-infection model | [63] |
| Caerin 1.9 | GLFGVLGSIAKHVLPHVVPVIAEKL-NH2 | HIV and Neisseria lactamica | In vitro assay | [64] |
| Hs02 | KWAVRIIRKFIKGFIS-NH2 (intragenic antimicrobial peptide-IAP) | P. aeruginosa and S. aureus | In vitro co-infection model | [65] |
| guanylated polymethacrylates | synthetic structural mimics of AMPs | C. albicans and S. aureus | In vitro co-infection model | [66] |
| Peptoid 5, 7 and 17 | poly-N-substituted glycines | C. albicans and S. aureus or C. albicans and E. coli | In vitro co-infection model | [67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batoni, G.; Maisetta, G.; Esin, S. Therapeutic Potential of Antimicrobial Peptides in Polymicrobial Biofilm-Associated Infections. Int. J. Mol. Sci. 2021, 22, 482. https://doi.org/10.3390/ijms22020482
Batoni G, Maisetta G, Esin S. Therapeutic Potential of Antimicrobial Peptides in Polymicrobial Biofilm-Associated Infections. International Journal of Molecular Sciences. 2021; 22(2):482. https://doi.org/10.3390/ijms22020482
Chicago/Turabian StyleBatoni, Giovanna, Giuseppantonio Maisetta, and Semih Esin. 2021. "Therapeutic Potential of Antimicrobial Peptides in Polymicrobial Biofilm-Associated Infections" International Journal of Molecular Sciences 22, no. 2: 482. https://doi.org/10.3390/ijms22020482