The Impact of Multiple Functional Layers in the Structure of Magnetic Nanoparticles and Their Influence on Albumin Interaction
Abstract
:1. Introduction
2. Results and Discussion
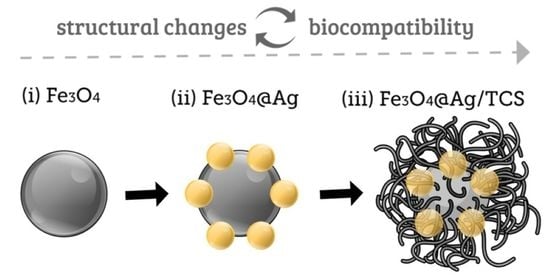
2.1. Morphological Characterization of Fe3O4 NPs, Fe3O4@Ag NPs, and Fe3O4@Ag/TCS NPs
2.2. Evaluation of the Fe3O4 Polymorphs in the NP
2.3. Interaction between NPs and Albumin
2.3.1. Influence of Albumin Adsorption on the Surface Properties of NPs
2.3.2. Determination of the Type of Complex between NPs and Albumin
2.3.3. Determination of Interaction Affinity between NPs and Albumin
2.3.4. Determination of Type of Interaction between NPs and Albumin
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of Fe3O4, Fe3O4@Ag NPs, Fe3O4@Ag/TCS NPs
3.3. Characterization of Fe3O4, Fe3O4@Ag NPs, and Fe3O4@Ag/TCS NPs
3.3.1. Morphological Characterizations
3.3.2. Mössbauer Spectroscopy
3.4. Interaction between NPs and Albumin
3.4.1. Influence of Albumin Adsorption on Fe3O4, Fe3O4@Ag NPs, and Fe3O4@Ag/TCS
3.4.2. Spectrofluorometric Analyses of the Interaction between NPs and Albumin
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanchez, L.M.; Alvarez, V.A. Advances in Magnetic Noble Metal/Iron-Based Oxide Hybrid Nanoparticles as Biomedical Devices. Bioengineering 2019, 6, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, X.; Zhang, L.; Yang, M.; Bai, L.; Dai, Y.; Yu, Z.; Pan, Y. Functional magnetic hybrid nanomaterials for biomedical diagnosis and treatment. Interdiscip. Rev. Nanomed Nanobiotechnol. 2018, 10, e1476. [Google Scholar] [CrossRef] [PubMed]
- Pieretti, J.C.; Rolim, W.R.; Ferreira, F.F.; Lombello, C.B.; Nascimento, M.H.M.; Seabra, A.B. Synthesis, Characterization, and Cytotoxicity of Fe3O4@Ag Hybrid Nanoparticles: Promising Applications in Cancer Treatment. J. Clust. Sci. 2020, 31, 535–547. [Google Scholar] [CrossRef]
- Rajkumar, S.; Prabaharan, M. Theranostic Application of Fe3O4-Au Hybrid Nanoparticles. In Noble Metal-Metal Oxide Hybrid Nanoparticles Fundamentals and Applications; Elsevier: New York, NY, USA, 2018; pp. 607–623. [Google Scholar]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Hayashi, K. Review Multifunctional silica-based hybrid nanoparticles for biomedical applications. J. Ceram. Soc. Jpn. 2016, 124, 855–862. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Shang, W.; Sun, X.; Zhao, L.; Wang, J.; Xiong, Z.; Yuan, J.; Zhang, R.; Huang, Q.; Wang, K.; et al. “All-in-One” Nanoparticles for Trimodality Imaging-Guided Intracellular Photo-magnetic Hyperthermia Therapy under Intravenous Administration. Adv. Funct. Mater. 2018, 28, 1705710. [Google Scholar] [CrossRef]
- Aggarwal, P.; Hall, J.B.; McLeland, C.B.; Dobrovolskaia, M.A.; McNeil, S.E. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv. Drug Deliv. Rev. 2009, 61, 428–437. [Google Scholar] [CrossRef] [Green Version]
- Saptarshi, S.R.; Duschl, A.; Lopata, A.L. Interaction of nanoparticles with proteins: Relation to bio-reactivity of the nanoparticle. J. Nanobiotechnol. 2013, 11, 26. [Google Scholar] [CrossRef] [Green Version]
- Gunawan, C.; Lim, M.; Marquis, C.P.; Amal, R. Nanoparticle-protein corona complexes govern the biological fates and functions of nanoparticles. J. Mater. Chem. B 2014, 2, 2060–2083. [Google Scholar] [CrossRef]
- Gao, H.; He, Q. The interaction of nanoparticles with plasma proteins and the consequent influence on nanoparticles behavior. Expert Opin. Drug Deliv. 2014, 11, 409–420. [Google Scholar] [CrossRef]
- Pieretti, J.C.; Pelegrino, M.T.; Nascimento, M.H.M.; Tortella, G.R.; Rubilar, O.; Seabra, A.B. Small molecules for great solutions: Can nitric oxide-releasing nanomaterials overcome drug resistance in chemotherapy? Biochem. Pharmacol. 2020, 176, 113740. [Google Scholar] [CrossRef]
- Marozkina, N.; Gaston, B. An Update on Thiol Signaling: S-Nitrosothiols, Hydrogen Sulfide and a Putative Role for Thionitrous Acid. Antioxidants 2020, 9, 225. [Google Scholar] [CrossRef] [Green Version]
- Anitha, A.; Deepa, N.; Chennazhi, K.P.; Nair, S.V.; Tamura, H.; Jayakumar, R. Development of mucoadhesive thiolated chitosan nanoparticles for biomedical applications. Carbohydr. Polym. 2011, 83, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Rolim, W.R.; Pelegrino, M.T.; Lima, B.A.; Ferraz, L.S.; Costa, F.N.; Bernardes, J.S.; Rodigues, T.; Brocchi, M.; Seabra, A.B. Green tea extract mediated biogenic synthesis of silver nanoparticles: Characterization, cytotoxicity evaluation and antibacterial activity. Appl. Surf. Sci. 2019, 463, 66–74. [Google Scholar] [CrossRef]
- Park, E.J.; Yi, J.; Kim, Y.; Choi, K.; Park, K. Silver nanoparticles induce cytotoxicity by a Trojan-horse type mechanism. Toxicol. Vitr. 2010, 24, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Schwaminger, S.P.; Bauer, D.; Fraga-García, P.; Wagner, F.E.; Berensmeier, S. Oxidation of magnetite nanoparticles: Impact on surface and crystal properties. CrystEngComm 2017, 19, 246–255. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Gupta, A.; Verma, N.C.; Nandi, C.K. Kinetics of protein adsorption on gold nanoparticle with variable protein structure and nanoparticle size. J. Chem. Phys. 2015, 143, 164709. [Google Scholar] [CrossRef]
- Rochani, A.K.; Balasubramanian, S.; Ravindran Girija, A.; Maekawa, T.; Kaushal, G.; Kumar, D.S. Heat Shock Protein 90 (Hsp90)-Inhibitor-Luminespib-Loaded-Protein-Based Nanoformulation for Cancer Therapy. Polymers 2020, 12, 1798. [Google Scholar] [CrossRef]
- Desai, N. Nanoparticle albumin-bound paclitaxel (Abraxane®). In Albumin in Medicine; Springer: Singapore, 2016; pp. 101–119. [Google Scholar]
- Yang, Q.; Liang, J.; Han, H. Probing the Interaction of Magnetic Iron Oxide Nanoparticles with Bovine Serum Albumin by Spectroscopic Techniques. J. Phys. Chem. B 2009, 113, 10454–10458. [Google Scholar] [CrossRef]
- Sun, J.; Shi, H.; Mo, T.; Zhang, Y.; Wang, X.; Ding, C.; Yu, S. Protein Binding on the Surface of Magnetic Nanoparticles. Part. Part. Syst. Charact. 2019, 36, 1900072. [Google Scholar] [CrossRef]
- Umerska, A.; Sapin-Minet, A.; Parent, M.; Tajber, L.; Maincent, P.; Boudier, A. Understanding the Thermodynamic Mechanisms Leading to the Binding of Albumin to Lipid Nanocapsules. Langmuir 2020, 36, 4165–4173. [Google Scholar] [CrossRef]
- Sangrà, M.; Estelrich, J.; Sabaté, R.; Espargaró, A.; Busquets, M.A. Evidence of protein adsorption in pegylated liposomes: Influence of liposomal decoration. Nanomaterials 2017, 7, 37. [Google Scholar] [CrossRef] [Green Version]
- Pieretti, J.C.; Gonçalves, M.C.; Nakazato, G.; de Souza, A.C.S.; Boudier, A.; Seabra, A.B. Multifunctional hybrid nanoplatform based on Fe3O4@Ag NPs for nitric oxide delivery: Development, characterization, therapeutic efficacy, and hemocompatibility. J. Mater. Sci. Mater. Med. 2021, 32, 23. [Google Scholar] [CrossRef]
- Kurtz-Chalot, A.; Villiers, C.; Pourchez, J.; Boudard, D.; Martini, M.; Marche, P.N.; Cottier, M.; Forest, V. Impact of silica nanoparticle surface chemistry on protein corona formation and consequential interactions with biological cells. Mater. Sci. Eng. C 2017, 75, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Guo, H.; Liu, L.; Liu, Y.; Xie, L. Size-dependent cellular uptake and localization profiles of silver nanoparticles. Int. J. Nanomed. 2019, 14, 4247–4259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Illés, E.; Tombácz, E. The effect of humic acid adsorption on pH-dependent surface charging and aggregation of magnetite nanoparticles. J. Colloid Interface Sci. 2006, 295, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Suh, C.Y.; Cho, S.W.; Roh, K.M.; Kwon, H.; Song, K.; Shon, I.J. A new method for the identification and quantification of magnetite-maghemite mixture using conventional X-ray diffraction technique. Talanta 2012, 94, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Percheron, A.; Chaumont, D.; Brachais, C.-H. Microwave-assisted one-step hydrothermal synthesis of pure iron oxide nanoparticles: Magnetite, maghemite and hematite. J. Sol-Gel Technol. 2011, 60, 198–205. [Google Scholar] [CrossRef]
- Dézsi, I.; Fetzer, C.; Gombköt, Á.; Szucs, I.; Gubicza, J.; Ungár, T. Phase transition in nanomagnetite. J. Appl. Phys. 2008, 103, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Masthoff, I.C.; Kraken, M.; Mauch, D.; Menzel, D.; Munevar, J.A.; Baggio Saitovitch, E.; Litterst, F.J.; Garnweitner, G. Study of the growth process of magnetic nanoparticles obtained via the non-aqueous sol-gel method. J. Mater. Sci. 2014, 49, 4705–4714. [Google Scholar] [CrossRef]
- Fock, J.; Bogart, L.K.; González-Alonso, D.; Espeso, J.I.; Hansen, M.F.; Varón, M.; Frandsen, C.; Pankhurst, Q.A. On the “centre of gravity” method for measuring the composition of magnetite/maghemite mixtures, or the stoichiometry of magnetite-maghemite solid solutions, via 57Fe Mössbauer spectroscopy. J. Phys. D Appl. Phys. 2017, 50, 265005. [Google Scholar] [CrossRef]
- Saikia, J.; Yazdimamaghani, M.; Pouya, S.; Moghaddam, H.; Ghandehari, H. Differential Protein Adsorption and Cellular Uptake of Silica Nanoparticles Based on Size and Porosity. ACS Appl. Mater. Interfaces 2016, 8, 34820–34832. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, D.C.; Qian, H.; Gies, V.; Yang, L. Human serum albumin stabilizes aqueous silver nanoparticle suspensions and inhibits particle uptake by cells. Environ. Sci. Nano 2018, 5, 863–867. [Google Scholar]
- Azizi, M.; Ghourchian, H.; Yazdian, F.; Dashtestani, F.; Alizadehzeinabad, H. Cytotoxic effect of albumin coated copper nanoparticle on human breast cancer cells of MDA-MB-231. PLoS ONE 2017, 12, e0188639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, S.; Ding, L.; Tian, Y.; Song, D.; Zhou, X.; Liu, X.; Zhang, H. Investigation of the interaction between flavonoids and human serum albumin. J. Mol. Struct. 2004, 703, 37–45. [Google Scholar] [CrossRef]
- Derakhshankhah, H.; Hosseini, A.; Taghavi, F.; Jafari, S.; Lotfabadi, A.; Ejtehadi, M.R.; Shahbazi, S.; Fattahi, A.; Ghasemi, A.; Barzegari, E.; et al. Molecular interaction of fibrinogen with zeolite nanoparticles. Sci. Rep. 2019, 9, 1–14. [Google Scholar]
- Paul, B.K.; Bhattacharjee, K.; Bose, S.; Guchhait, N. A spectroscopic investigation on the interaction of a magnetic ferrofluid with a model plasma protein: Effect on the conformation and activity of the protein. Phys. Chem. Chem. Phys. 2012, 14, 15482–15493. [Google Scholar] [CrossRef] [PubMed]
- Pallotta, A.; Parent, M.; Clarot, I.; Luo, M.; Borr, V.; Dan, P.; Decot, V.; Menu, P.; Safar, R.; Joubert, O.; et al. Blood Compatibility of Multilayered Polyelectrolyte Films Containing Immobilized Gold Nanoparticles. Part. Part. Syst. Charact. 2017, 34, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Beurton, J.; Lavalle, P.; Pallotta, A.; Chaigneau, T.; Clarot, I.; Boudier, A. Design of surface ligands for blood compatible gold nanoparticles: Effect of charge and binding energy. Int. J. Pharm. 2020, 580, 119244. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, G.M.; Butcher, N.J.; Musumeci, A.W.; Deng, Z.J.; Martin, D.J.; Minchin, R.F. Cryptic epitopes of albumin determine mononuclear phagocyte system clearance of nanomaterials. ACS Nano 2014, 8, 3357–3366. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Shokrgozar, M.A.; Sardari, S.; Moghadam, M.K.; Vali, H.; Laurent, S.; Stroeve, P. Irreversible changes in protein conformation due to interaction with superparamagnetic iron oxide nanoparticles. Nanoscale 2011, 3, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.D.; Subramanian, S. Thermodynamics of macromolecular association reactions: Analysis of forces contributing to stabilization. Biophys. J. 1980, 32, 79–81. [Google Scholar] [CrossRef] [Green Version]
- Rolim, W.R.; Pieretti, J.C.; Renó, D.L.S.; Lima, B.A.; Nascimento, M.H.M.; Ambrosio, F.N.; Lombello, C.B.; Brocchi, M.; De Souza, A.C.S.; Seabra, A.B. Antimicrobial Activity and Cytotoxicity to Tumor Cells of Nitric Oxide Donor and Silver Nanoparticles Containing PVA/PEG Films for Topical Applications. ACS Appl. Mater. Interfaces 2019, 11, 6589–6604. [Google Scholar] [CrossRef]
- Vroman, L. Effect of Adsorbed Proteins on the Wettability of Hydrophilic and Hydrophobic Solids. Nature 1962, 196, 476–477. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, T.I.; Daves, M.C.; Dium, L. Human Serum Albumin as a Probe for Protein Adsorption to Nanopar-tides: Relevance to Biodistribution. J. Drug Target. 1997, 4, 389–398. [Google Scholar] [CrossRef]





| Fe3O4 NPs | ||||
|---|---|---|---|---|
| δ (mm/s) | ΔEQ (mm/s) | BHF (T) | % Area | |
| Site 1 | 0.453(7) | −0.06(2) | 50.3(1) | 42.0 |
| Site 2 | −0.08(4) | 41.4(2) | 10.9 | |
| Site 3 | −0.04(7) | 29.7(2) | 9.9 | |
| Site 4 | 0.446(9) | −0.08(2) | 47.2(1) | 22.5 |
| Site 5 | 0.13(8) | 20.6(3) | 7.0 | |
| Site 6 | 0.11(7) | 8.1(2) | 7.6 | |
| Fe3O4@Ag NPs | ||||
| Site 1 | 0.451(5) | 0.00(1) | 52.2(1) | 59.4 |
| Site 2 | −0.11(20) | 30.5(5) | 3.2 | |
| Site 3 | 0.426(9) | −0.03(2) | 49.7(1) | 27.0 |
| Site 4 | 0.46(36) | 20.6(1.4) | 1.1 | |
| Site 5 | 0.703(34) | 0.08(8) | 42.9(3) | 5.7 |
| Site 6 | −0.31(14) | 8.3(5) | 3.5 | |
| Fe3O4@Ag-TCS NPs | ||||
| Site 1 | 0.480(3) | 0.18(1) | 51.7(2) | 47.1 |
| Site 2 | 0.17(6) | 41.4(2) | 2.6 | |
| Site 3 | 0.386(4) | −0.23(1) | 51.2(1) | 36.9 |
| Site 4 | 0.54(5) | 30.3(2) | 3.5 | |
| Site 5 | 0.559(4) | 0.01(3) | 45.9(1) | 6.2 |
| Site 6 | −0.38(5) | 8.5(2) | 3.6 | |
| NP | T (K) | Ksv (L mol−1) | R2 | Kq (1010 mol−1 L−1) |
|---|---|---|---|---|
| Fe3O4 | 298 310 330 | 1.37 × 104 1.01 × 104 1.80 × 104 | 0.99 0.99 0.99 | 13.7 10.1 18.0 |
| Fe3O4@Ag | 298 310 330 | 1.14 × 104 1.23 × 104 1.59 × 104 | 0.98 0.97 0.92 | 11.4 12.3 15.9 |
| Fe3O4@Ag/TCS | 298 310 330 | 2.39 × 103 2.50 × 103 6.09 × 103 | 0.98 0.92 0.95 | 2.39 2.50 6.09 |
| NP | T (K) | Ka (L mol−1) | ∆H (kJ mol−1) | ∆S (J mol−1 K−1) | ∆G (kJ mol−1) | Interaction Mechanism |
|---|---|---|---|---|---|---|
| Fe3O4 | 298 | 7.45 × 103 | 19.63 | 140.51 | −22.09 | Hydrophobic interactions |
| 310 | 7.72 × 103 | −23.08 | ||||
| 330 | 2.08 × 104 | −27.28 | ||||
| Fe3O4@Ag | 298 | 5.34 × 103 | 13.13 | 118.86 | −21.27 | Hydrophobic interactions |
| 310 | 7.83 × 103 | −23.11 | ||||
| 330 | 1.76 × 104 | −26.84 | ||||
| Fe3O4@Ag/TCS | 298 | 2.62 × 103 | 25.61 | 149.78 | −19.51 | Hydrophobic interactions |
| 310 | 1.93 × 103 | −19.50 | ||||
| 330 | 7.29 × 103 | −24.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pieretti, J.C.; Beurton, J.; Munevar, J.; Nagamine, L.C.C.M.; Le Faou, A.; Seabra, A.B.; Clarot, I.; Boudier, A. The Impact of Multiple Functional Layers in the Structure of Magnetic Nanoparticles and Their Influence on Albumin Interaction. Int. J. Mol. Sci. 2021, 22, 10477. https://doi.org/10.3390/ijms221910477
Pieretti JC, Beurton J, Munevar J, Nagamine LCCM, Le Faou A, Seabra AB, Clarot I, Boudier A. The Impact of Multiple Functional Layers in the Structure of Magnetic Nanoparticles and Their Influence on Albumin Interaction. International Journal of Molecular Sciences. 2021; 22(19):10477. https://doi.org/10.3390/ijms221910477
Chicago/Turabian StylePieretti, Joana C., Jordan Beurton, Julián Munevar, Luiz C. C. M. Nagamine, Alain Le Faou, Amedea B. Seabra, Igor Clarot, and Ariane Boudier. 2021. "The Impact of Multiple Functional Layers in the Structure of Magnetic Nanoparticles and Their Influence on Albumin Interaction" International Journal of Molecular Sciences 22, no. 19: 10477. https://doi.org/10.3390/ijms221910477