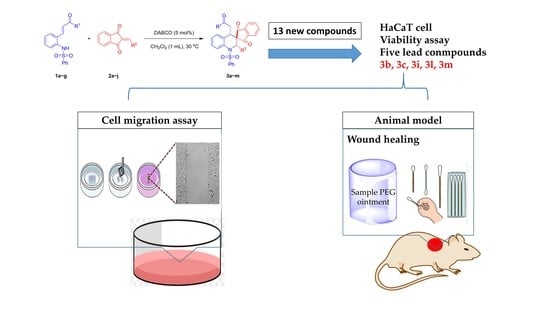
Synthesis of Novel Spiro-Tetrahydroquinoline Derivatives and Evaluation of Their Pharmacological Effects on Wound Healing
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Ortho-N-Protected Aminophenyl α,β-Unsaturated Ketones (1)
4.2. 2-Arylidene-1,3-Indandiones (2)
4.3. Spiro-Tetrahydroquinoline (3)
4.4. Cell Culture
4.5. Cell Viability Assay
4.6. Scratch Assay
4.7. Splints
4.8. Preparation of Sample-Containing PEG-Based Ointment
4.9. Animal Experiments
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TLC | thin layer chromatography |
| NMR | nuclear magnetic resonance |
| DABCO | 1:4-diazabicyclo[2.2.2]octane |
| DMAP | 4-dimethylaminopyridine |
| Et3N | triethylamine |
| DCM | dichloromethane |
| Et2O | ether |
| THF | tetrahydrofuran |
| EA | ethyl acetate |
| MeCN | acetonitrile |
| r.t. | room temperature |
| Mp | melting point |
| FBS | fetal bovine serum |
| MTT | methythiazolyltetrazolium |
| PEG | polyethylene glycol |
References
- Schulz, J.T., III; Tompkins, R.G.; Burke, J.F. Artificial Skin. Annu. Rev. Med. 2000, 51, 231. [Google Scholar] [CrossRef]
- Sun, G.; Mao, J.J. Engineering dextran-based scaffolds for drug delivery and tissue repair. Nanomedicine 2012, 1771–1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabine, W.; Richard, G. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef]
- Sosne, G.; Qiu, P.; Goldstein, A.L.; Wheater, M. Biological activities of thymosin beta4 defined by active sites in short peptide sequences. FASEB J. 2010, 24, 2144–2151. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, Y.; Zou, Z.; Yang, M.; Wu, C.; Su, Y.; Tang, J.; Yang, X. OM-LV20, a novel peptide from odorous frog skin, accelerates wound healing in vitro and in vivo. Chem. Biol. Drug Des. 2018, 91, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Arturson, G. Pathophysiology of the burn wound and pharmacological treatment. The Rudi Hermans Lecture, 1995. Burns 1996, 22, 255. [Google Scholar] [CrossRef]
- Kumar, S.; Bawa, S.; Gupta, H. Biological activities of quinoline derivatives. Mini-Rev. Med. Chem. 2009, 9, 1648–1654. [Google Scholar] [CrossRef]
- Kubica, K.; Taciak, P.; Czajkowska, A.; Ignasiak, A.S.; Wyrebiak, R.; Podsadni, P.; Bia£y, I.M.; Malejczyk, J.; Mazurek, A.P. Synthesis and anticancer activity evaluation of some new derivatives of 2-(4-benzoyl-1-piperazinyl)-quinoline and 2-(4-cinnamoyl-1-piperazinyl)-quinoline. Acta Pol. Pharm. Drug Res. 2018, 75, 891–901. [Google Scholar] [CrossRef]
- Li, W.; Shuai, W.; Sun, H.; Xu, F.; Bi, Y.; Xu, J.; Ma, C.; Yao, H.; Zhu, Z.; Xu, S. Design, synthesis and biological evaluation of quinoline-indole derivatives as anti-tubulin agents targeting the colchicine binding site. Eur. J. Med. Chem. 2019, 163, 428–442. [Google Scholar] [CrossRef] [PubMed]
- Prakash Naik, H. Bhojya Naik, H.; Ravikumar Naik, T.; Naika, H.; Gouthamchandra, K.; Mahmood, R.; Khadeer Ahamed, B. Synthesis of novel benzo[h]quinolines: Wound healing, antibacterial, DNA binding and in vitro antioxidant activity. Eur. J. Med. Chem. 2009, 44, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Teng, P.; Zou, Y.; Zhang, C.; Shen, X.; Cai, J.; Hu, Y. Small antimicrobial agents encapsulated in poly(epsilon-caprolactone)-poly(ethylene glycol) nanoparitcles for treatment of S. aureus-infected wounds. J. Nanopart. Res. 2018, 20, 270. [Google Scholar] [CrossRef]
- Goli, N.; Mainkar, P.; Kotapalli, S.; Tejaswini, K.; Ummanni, R.; Chandrasekhar, S. Expanding the tetrahydroquinoline pharmacophore. Bioorg. Med. Chem. Lett. 2017, 8, 1714–1720. [Google Scholar] [CrossRef] [PubMed]
- Mail kumaran, P.; Sheeja Devi, K.; Manikantha mouli, C.H.; Manjula, M.; Smylin Ajitha Rani, S.; Krishnamalar, G. Synthesis, characterization and wound healing activity of tetrazoloquinoline thiocarbohydrazide derivatives. Int. J. Res. Pharm. Nano Sci. 2012, 1, 70–79. [Google Scholar]
- Duan, J.D.; Cheng, J.; Li, P.F. Enantioselective Construction of Spiro-1,3-indandiones with Three Stereocenters via Organocatalytic Michael-Aldol Reaction of 2-Arylideneindane-1,3-diones and Nitro Aldehydes. Org. Chem. Front. 2015, 2, 1048–1052. [Google Scholar] [CrossRef]
- Singh, G.S. and Desta, Z.Y. Isatins as privileged molecules in design and synthesis of spiro-fused cyclic frameworks. Chem. Rev. 2012, 112, 6104. [Google Scholar] [CrossRef]
- Chai, Z.; Rainey, T.J. Pd(II)/brønsted acid catalyzed enantioselective allylic C–H activation for the synthesis of spiro-cyclic rings. J. Am. Chem. Soc. 2012, 134, 3615. [Google Scholar] [CrossRef]
- Pandey, R.C.; Toussaint, M.W.; Stroshane, R.M.; Kahta, C.C.; Aszalos, A.A.; Garretson, A.L.; Wei, T.T.; Byrne, K.M.; Geoghegan, R.F., Jr.; White, R.J.I. Fredericamycin A, a new antitumor antibiotic. I. Production, isolation and physicochemical properties. Antibiotics 1981, 34, 1389. [Google Scholar] [CrossRef]
- Clive, D.L.J.; Kong, X.L.; Paul, C.C. Further Model Studies Related to Fredericamycin A: Analogues in which Ring C is Expanded to Six Atoms, and an Examination of the Diastereoselectivity of Radical Spiro-cyclization. Tetrahedron 1996, 52, 6085–6116. [Google Scholar] [CrossRef]
- Evans, P.A.; Brandt, T.A. Palladium Catalyzed Cross-Coupling Acylation Approach to the Antitumor Antibiotic Fredericamycin A. Tetrahedron Lett. 1996, 37, 1367–1370. [Google Scholar] [CrossRef]
- Pizzirani, D.; Roberti, M.; Grimaudo, S.; Cristina, A.; Pipitone, R.M.; Tolomeo, M.; Recanatini, M. Identification of Biphenyl-Based Hybrid Molecules Able To Decrease the Intracellular Level of Bcl-2 Protein in Bcl-2 Overexpressing Leukemia Cells. J. Med. Chem. 2009, 52, 6936–6940. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Cao, W.; Tong, W.; Chen, J.; Deng, H.; Wu, D. Triphenylarsine-catalyzed cyclopropanation: Highly stereoselective synthesis of trans -2,3-dihydro-spiro[cyclopropane-1,2′-indan-1′,3′-dione] from alkene and phenacyl bromide. Synth. Commun. 2008, 38, 2200. [Google Scholar] [CrossRef]
- Russo, A.; Meninno, S.; Tedesco, C.; Lattanzi, A. Synthesis of Activated Cyclopropanes by an MIRC Strategy: An Enantioselective Organocatalytic Approach to Spiro-cyclopropanes. Eur. J. Org. Chem. 2011, 5096. [Google Scholar] [CrossRef]
- Lu, Y.L.; Sun, J.; Jiang, Y.H.; Yan, C.G. Diastereoselective synthesis of spiro [indene-2,20-pyrazolo [1,2-a] pyrazoles] and spiro [indoline-3,20-pyrazolo [1,2-a] pyrazoles] via 1,3-dipolar cycloaddition. RSC Adv. 2016, 6, 50471–50478. [Google Scholar] [CrossRef]
- Mahajan, S.; Chauhan, P.; Blümel, M.; Puttreddy, R.; Rissanen, K.; Raabe, G.; Enders, D. Asymmetric Synthesis of Spiro Tetrahydrothiophene-indan-1,3-diones via a Squaramide-Catalyzed Sulfa-Michael/Aldol Domino Reaction. Synthesis 2016, 48, 41131–41138. [Google Scholar] [CrossRef]
- Das, U.; Tsai, Y.L.; Lin, W. An efficient organocatalytic enantioselective synthesis of spiro-nitrocyclopropanes. Org. Biomol. Chem. 2013, 11, 44–47. [Google Scholar] [CrossRef]
- Madhusudhan Reddy, G.; Ko, C.T.; Hsieh, K.H.; Lee, C.J.; Das, U.; Lin, W. Expanding the Scope of Primary Amine Catalysis: Stereoselective Synthesis of Indanedione-Fused 2,6-Disubstituted trans-spiro-cyclohexanones. J. Org. Chem. 2016, 81, 2420–2431. [Google Scholar] [CrossRef]
- Lin, Y.; Chu, P.; Ma, W.; Cheng, W.; Chan, S.; Yang, J.; Wu, Y. Enzyme-digested peptides derived from Lates calcarifer enhance wound healing after surgical repair. Mar. Drug 2021, 19, 154. [Google Scholar] [CrossRef]
- Song, Y.X.; Du, D.M. Asymmetric synthesis of highly functionalized spirothiazolidinone tetrahydroquinolines via a squaramide-catalyzed cascade reaction. Org. Biomol. Chem. 2018, 16, 9390–9401. [Google Scholar] [CrossRef]
- X-ray Crystallographic Data of Compound CCDC 1863526. Available online: www.ccdc.cam.ac.uk/data_request/cif (accessed on 1 May 2021).
- Liu, S.P.; Shibu, M.A.; Tsai, F.J.; Hsu, Y.M.; Tsai, C.H.; Chung, J.G.; Yang, J.S.; Tang, C.H.; Wang, S.; Li, Q.; et al. Tetramethylpyrazine reverses high-glucose induced hypoxic effects by negatively regulating HIF-1alpha induced BNIP3 expression to ameliorate H9c2 cardiomyoblast apoptosis. Nutr. Metab. 2020, 17, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.P.; Wang, S.W.; Wu, Y.C.; Tsai, C.H.; Tsai, F.J.; Chung, J.G.; Huang, C.Y.; Yang, J.S.; Hsu, Y.M.; Yin, M.C.; et al. Glucocerebroside reduces endothelial progenitor cell-induced angiogenesis. Food Agric. Immunol. 2019, 30, 1033–1045. [Google Scholar] [CrossRef] [Green Version]
- Koo, Y.M.; Yun, Y.H. Effects of polydeoxyribonucleotides (PDRN) on wound healing: Electric cell-substrate impedance sensing (ECIS). Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Chen, K.B.; Tsai, C.H.; Tsai, F.J.; Huang, C.Y.; Tang, C.H.; Yang, J.S.; Hsu, Y.M.; Peng, S.F.; Chung, J.-G. Casticin inhibits human prostate cancer DU 145 cell migration and invasion via Ras/Akt/NF-κB signaling pathways. J. Food Biochem. 2019, 43, e12902. [Google Scholar] [CrossRef]
- Jimi, S.; Francesco, D.F.; Ferraro, G.; Riccio, M.; Hara, S. A Novel Skin Splint for Accurately Mapping Dermal Remodeling and Epithelialization during Wound Healing. J. Cell Physiol. 2016, 232, 1225–1232. [Google Scholar] [CrossRef]
- Marshall, C.D.; Hu, M.S.; Leavitt, T.; Barnes, L.A.; Cheung, A.T.; Malhotra, S.; Lorenz, H.P.; Delp, S.L.; Quake, S.R.; Longaker, M.T. Sanativo Wound Healing Product Does Not Accelerate Reepithelialization in a Mouse Cutaneous Wound Healing Model. Plast. Reconstr. Surg. 2017, 139, 343–352. [Google Scholar] [CrossRef]
- Kang, Y.K.; Lee, Y.M.; Im, S.; Park, H.; Kim, W.J. Nitric oxide-releasing polymer incorporated ointment for cutaneous wound healing. J. Control. Release 2015, 220, 624–630. [Google Scholar] [CrossRef]








| Entry | Cat. | Solvent | Temp. (°C) | Time (h) | Yield (%) b,c |
|---|---|---|---|---|---|
| 1 | DABCO | p-Xylene | 30 | 12 | 71 |
| 2 | DMAP | p-Xylene | 30 | 12 | 47 |
| 3 | NEt3 | p-Xylene | 30 | 24 | 57 |
| 4 | DIPEA | p-Xylene | 30 | 24 | 17 |
| 5 | DABCO | Toluene | 30 | 12 | 91 |
| 6 | DABCO | CH2Cl2 | 30 | 12 | 96 |
| 7 | DABCO | THF | 30 | 12 | 77 |
| 8 | DABCO | EtOAc | 30 | 12 | 88 |
| 9 | DABCO | MeCN | 30 | 12 | 87 |
| 10 | DABCO | Et2O | 30 | 12 | 11 |
| 11 | DABCO | CH2Cl2 | 0 | 38 | 97 |
| 12 d,e | DABCO | CH2Cl2 | 30 | 12 | 90 |
| 13 d,f | DABCO | CH2Cl2 | 30 | 12 | 92 |

| Entry | R1 | R2 | Time (h) | Yield (%) b |
|---|---|---|---|---|
| 1 | Ph (1a) | Ph (2a) | 12 | 92 (3a) |
| 2 | Ph (1a) | 2-BrPh (2b) | 24 | 71 (3b) |
| 3 | Ph (1a) | 3-BrPh (2c) | 3 | 81 (3c) |
| 4 | Ph (1a) | 4-BrPh (2d) | 3 | 83 (3d) |
| 5 | Ph (1a) | 4-ClPh (2e) | 3 | 86 (3e) |
| 6 | Ph (1a) | 4-CNPh (2f) | 3 | 81 (3f) |
| 7 | Ph (1a) | 4-NO2Ph (2g) | 3 | 82 (3g) |
| 8 | Ph (1a) | 4-OMePh (2h) | 29 | 68 (3h) |
| 9 | Ph (1a) | Furyl (2i) | 24 | N.R. |
| 10 | Ph (1a) | Thienyl (2j) | 24 | N.R. |
| 11 | H (1b) | Ph (2a) | 3 | 95 (3i) |
| 12 | 4-BrPh (1c) | Ph (2a) | 3 | 93 (3j) |
| 13 | 4-NO2Ph (1d) | Ph (2a) | 3 | 96 (3k) |
| 14 | 4-OMePh (1e) | Ph (2a) | 12 | 89 (3l) |
| 15 | OEt (1f) | Ph (2a) | 24 | N.R. |
| 16 | 2-Naph (1g) | Ph (2a) | 6 | 85 (3m) |
| Cell Viability after 24 Hours (% Cell Viability) a | |||||
|---|---|---|---|---|---|
| Compounds | 6.25 | 12.5 | 25 | 50 | 100 |
| 3a | 51.85 ± 1.52 | 46.53 ± 1.73 | 45.02 ± 2.07 | 39.66 ± 0.94 | 36.15 ± 0.91 |
| 3b | 91.58 ± 3.00 | 89.56 ± 4.50 | 86.63 ± 3.02 | 80.75 ± 2.61 | 75.33 ± 2.27 |
| 3c | 92.63 ± 1.89 | 75.38 ± 3.36 | 66.05 ± 0.57 | 54.61 ± 2.84 | 44.72 ± 4.14 |
| 3d | 71.53 ± 3.02 | 55.22 ± 4.37 | 45.98 ± 0.63 | 41.35 ± 2.36 | 35.24 ± 0.86 |
| 3e | 59.63 ± 3.10 | 51.38 ± 3.10 | 47.06 ± 1.18 | 42.13 ± 1.18 | 37.55 ± 1.78 |
| 3f | 43.08 ± 2.68 | 38.78 ± 3.54 | 39.22 ± 3.23 | 35.45 ± 2.68 | 27.08 ± 6.40 |
| 3g | 49.53 ± 0.63 | 43.68 ± 1.41 | 40.21 ± 1.04 | 38.91 ± 0.60 | 33.18 ± 1.32 |
| 3h | 73.02 ± 3.04 | 65.79 ± 0.77 | 62.66 ± 0.96 | 52.92 ± 0.76 | 43.04 ± 1.19 |
| 3i | 87.90 ± 5.68 | 61.38 ± 6.78 | 18.21 ± 4.26 | 14.23 ± 1.10 | 14.10 ± 1.69 |
| 3j | 40.36 ± 1.71 | 26.33 ± 0.92 | 19.02 ± 0.40 | 15.73 ± 0.47 | 11.45 ± 0.11 |
| 3k | 20.09 ± 0.64 | 7.05 ± 0.17 | 7.15 ± 0.07 | 7.03 ± 0.15 | 7.09 ± 0.13 |
| 3l | 87.31 ± 0.95 | 84.57 ± 1.78 | 82.97 ± 1.22 | 77.60 ± 1.43 | 76.15 ± 2.13 |
| 3m | 84.42 ± 1.69 | 70.56 ± 1.24 | 59.69 ± 2.02 | 47.18 ± 1.34 | 35.47 ± 0.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liou, Y.-C.; Lin, Y.-A.; Wang, K.; Yang, J.-C.; Jang, Y.-J.; Lin, W.; Wu, Y.-C. Synthesis of Novel Spiro-Tetrahydroquinoline Derivatives and Evaluation of Their Pharmacological Effects on Wound Healing. Int. J. Mol. Sci. 2021, 22, 6251. https://doi.org/10.3390/ijms22126251
Liou Y-C, Lin Y-A, Wang K, Yang J-C, Jang Y-J, Lin W, Wu Y-C. Synthesis of Novel Spiro-Tetrahydroquinoline Derivatives and Evaluation of Their Pharmacological Effects on Wound Healing. International Journal of Molecular Sciences. 2021; 22(12):6251. https://doi.org/10.3390/ijms22126251
Chicago/Turabian StyleLiou, Yan-Cheng, Yan-An Lin, Ke Wang, Juan-Cheng Yang, Yeong-Jiunn Jang, Wenwei Lin, and Yang-Chang Wu. 2021. "Synthesis of Novel Spiro-Tetrahydroquinoline Derivatives and Evaluation of Their Pharmacological Effects on Wound Healing" International Journal of Molecular Sciences 22, no. 12: 6251. https://doi.org/10.3390/ijms22126251