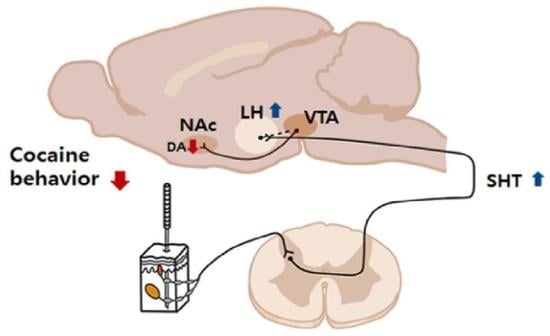
Role of Lateral Hypothalamus in Acupuncture Inhibition of Cocaine Psychomotor Activity
Abstract
:1. Introduction
2. Results
2.1. The LH Mediates the Inhibitory Effects of Acupuncture at HT7 on Cocaine-Induced Locomotor Activity
2.2. The LH Mediates the Inhibitory Effects of Acupuncture at HT7 on Cocaine-Induced 50-kHz USVs
2.3. The LH Mediates HT7 Inhibition of Cocaine-Induced DA Release in NAc
2.4. Acupuncture at HT7 Activates the LH and SHT Neurons
2.5. Activation of the LH Suppresses Cocaine-Induced Locomotor Activity
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Chemicals
4.3. Acupuncture Treatment
4.4. Pharmacological Lesion of Lateral Hypothalamus (LH)
4.5. Cocaine-Induced Locomotor Activity
4.6. Measurement of Ultrasonic Vocalizations
4.7. In Vivo Fast-Scan Cyclic Voltammetry (FSCV) for DA Detection
4.8. In Vivo Extracellular Recording of LH or Spinohypothalamic (SHT) Neurons
4.9. Activation of the LH by PEPA Microinjection
4.10. Immunohistochemistry for c-Fos
4.11. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Motlagh, F.E.; Ibrahim, F.; Rashid, R.A.; Seghatoleslam, T.; Habil, H. Acupuncture therapy for drug addiction. Chin. Med. 2016, 11, 16. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Programme on Traditional Medicine. In WHO Traditional Medicine Strategy 2002–2005; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Yoon, S.S.; Yang, E.J.; Lee, B.H.; Jang, E.Y.; Kim, H.Y.; Choi, S.-M.; Steffensen, S.C.; Yang, C.H. Effects of acupuncture on stress-induced relapse to cocaine-seeking in rats. Psychopharmacology 2012, 222, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Jin, W.; Kim, M.S.; Jang, E.Y.; Lee, J.Y.; Lee, J.G.; Kim, H.Y.; Yoon, S.S.; Lee, B.H.; Chang, S.; Kim, J.H.; et al. Acupuncture reduces relapse to cocaine-seeking behavior via activation of GABA neurons in the ventral tegmental area. Addict. Biol. 2018, 23, 165–181. [Google Scholar] [CrossRef]
- Kim, M.R.; Kim, S.J.; Lyu, Y.S.; Kim, S.H.; Lee, Y.K.; Kim, T.H.; Shim, I.; Zhao, R.; Golden, G.T.; Yang, C.H. Effect of acupuncture on behavioral hyperactivity and dopamine release in the nucleus accumbens in rats sensitized to morphine. Neurosci. Lett. 2005, 387, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.S.; Kwon, Y.K.; Kim, M.R.; Shim, I.; Kim, K.J.; Lee, M.H.; Lee, Y.S.; Golden, G.T.; Yang, C.H. Acupuncture-mediated inhibition of ethanol-induced dopamine release in the rat nucleus accumbens through the GABAB receptor. Neurosci. Lett. 2004, 369, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Kim, D.H.; Jang, E.Y.; Yoon, S.S.; Gwak, Y.S.; Yi, Y.J.; Lee, J.Y.; Ahn, S.H.; Kim, J.M.; Ryu, Y.-H.; et al. Acupuncture attenuates alcohol dependence through activation of endorphinergic input to the nucleus accumbens from the arcuate nucleus. Sci. Adv. 2019, 5, eaax1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.A.; Lee, B.H.; Bae, J.H.; Kim, K.J.; Steffensen, S.C.; Ryu, Y.-H.; Leem, J.; Yang, C.H.; Kim, H.Y. Peripheral Afferent Mechanisms Underlying Acupuncture Inhibition of Cocaine Behavioral Effects in Rats. PLoS ONE 2013, 8, e81018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyree, S.M.; de Lecea, L. Lateral hypothalamic control of the ventral tegmental area: Reward evaluation and the driving of motivated behavior. Front. Syst. Neurosci. 2017, 11, 50. [Google Scholar] [CrossRef] [Green Version]
- Dayas, C.V.; McGranahan, T.M.; Martin-Fardon, R.; Weiss, F. Stimuli Linked to Ethanol Availability Activate Hypothalamic CART and Orexin Neurons in a Reinstatement Model of Relapse. Biol. Psychiatry 2008, 63, 152–157. [Google Scholar] [CrossRef]
- Yeoh, J.W.; James, M.H.; Jobling, P.; Bains, J.S.; Graham, B.A.; Dayas, C.V. Cocaine potentiates excitatory drive in the perifornical/lateral hypothalamus. J. Physiol. 2012, 590, 3677–3689. [Google Scholar] [CrossRef]
- Harris, G.C.; Wimmer, M.; Aston-Jones, G. A role for lateral hypothalamic orexin neurons in reward seeking. Nat. Cell Biol. 2005, 437, 556–559. [Google Scholar] [CrossRef]
- Stamatakis, A.M.; Van Swieten, M.; Basiri, M.L.; Blair, G.A.; Kantak, P.; Stuber, G.D. Lateral Hypothalamic Area Glutamatergic Neurons and Their Projections to the Lateral Habenula Regulate Feeding and Reward. J. Neurosci. 2016, 36, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Lecca, S.; Meye, F.J.; Trusel, M.; Tchenio, A.; Harris, J.; Schwarz, M.K.; Burdakov, D.; Georges, F.; Mameli, M. Aversive stimuli drive hypothalamus-to-habenula excitation to promote escape behavior. eLife 2017, 6, e30697. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Gokin, A.P.; Giesler, G.J., Jr. Responses of spinohypothalamic tract neurons in the thoracic spinal cord of rats to somatic stimuli and to graded distention of the bile duct. Somatosens. Mot. Res. 2002, 19, 5–17. [Google Scholar] [CrossRef]
- Dado, R.J.; Katter, J.T.; Giesler, G.J., Jr. Spinothalamic and spinohypothalamic tract neurons in the cervical enlargement of rats. II. Responses to innocuous and noxious mechanical and thermal stimuli. J. Neurophysiol. 1994, 71, 981–1002. [Google Scholar] [CrossRef]
- Burstein, R.; Dado, R.J.; Cliffer, K.D.; Giesler, G.J., Jr. Physiological characterization of spinohypothalamic tract neurons in the lumbar enlargement of rats. J. Neurophysiol. 1991, 66, 261–284. [Google Scholar] [CrossRef]
- Stuber, G.D.; Wise, R.A. Lateral hypothalamic circuits for feeding and reward. Nat. Neurosci. 2016, 19, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Marchant, N.J.; Millan, E.Z.; McNally, G.P. The hypothalamus and the neurobiology of drug seeking. Cell. Mol. Life Sci. 2011, 69, 581–597. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Han, S.-M.; Shim, I. Acupuncture attenuates cocaine-induced expression of behavioral sensitization in rats: Possible involvement of the dopaminergic system in the ventral tegmental area. Neurosci. Lett. 2009, 449, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.; Yang, C.H.; Kwon, Y.K.; Kim, M.R.; Pyun, K.-H.; Hahm, D.-H.; Lee, H.-J.; Shim, I. Acupuncture attenuates repeated nicotine-induced behavioral sensitization and c-Fos expression in the nucleus accumbens and striatum of the rat. Neurosci. Lett. 2004, 358, 87–90. [Google Scholar] [CrossRef]
- Lee, B.H.; Ma, J.H.; In, S.; Kim, H.Y.; Yoon, S.S.; Jang, E.Y.; Yang, C.H. Acupuncture at SI5 attenuates morphine seeking behavior after extinction. Neurosci. Lett. 2012, 529, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Simola, N. Rat Ultrasonic Vocalizations and Behavioral Neuropharmacology: From the Screening of Drugs to the Study of Disease. Curr. Neuropharmacol. 2015, 13, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Bills, K.B.; Obray, J.; Clarke, T.; Parsons, M.; Brundage, J.; Yang, C.H.; Kim, H.Y.; Yorgason, J.T.; Blotter, J.D.; Steffensen, S.C. Mechanical stimulation of cervical vertebrae modulates the discharge activity of ventral tegmental area neurons and dopamine release in the nucleus accumbens. Brain Stimul. 2020, 13, 403–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, C.S.; Jeong, H.-S.; Park, H.-J.; Baik, Y.; Yoon, M.-H.; Choi, C.-B.; Koh, H.G. A proposed transpositional acupoint system in a mouse and rat model. Res. Veter-Sci. 2008, 84, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Ryu, Y.; Gwak, Y.S.; Kim, N.J.; Kim, J.M.; Lee, J.Y.; Kim, S.A.; Lee, B.H.; Steffensen, S.C.; Jang, E.Y.; et al. Spinal pathways involved in somatosensory inhibition of the psychomotor actions of cocaine. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Paxinos, G.; Watson, C.R.; Emson, P.C. AChE-stained horizontal sections of the rat brain in stereotaxic coordinates. J. Neurosci. Methods 1980, 3, 129–149. [Google Scholar] [CrossRef]
- Kim, N.J.; Ryu, Y.; Lee, B.H.; Chang, S.; Fan, Y.; Gwak, Y.S.; Yang, C.H.; Bills, K.B.; Steffensen, S.C.; Koo, J.S.; et al. Acupuncture inhibition of methamphetamine-induced behaviors, dopamine release and hyperthermia in the nucleus accumbens: Mediation of group II mGluR. Addict. Biol. 2019, 24, 206–217. [Google Scholar] [CrossRef]
- Jang, E.Y.; Ryu, Y.-H.; Lee, B.H.; Chang, S.-C.; Yeo, M.J.; Kim, S.H.; Folsom, R.J.; Schilaty, N.D.; Kim, K.J.; Yang, C.H.; et al. Involvement of reactive oxygen species in cocaine-taking behaviors in rats. Addict. Biol. 2015, 20, 663–675. [Google Scholar] [CrossRef] [Green Version]
- Burstein, R.; Cliffer, K.D.; Giesler, G.J., Jr. Cells of origin of the spinohypothalamic tract in the rat. J. Comp. Neurol. 1990, 291, 329–344. [Google Scholar] [CrossRef]
- Huff, M.L.; Lalumiere, R.T. The Rostromedial Tegmental Nucleus Modulates Behavioral Inhibition Following Cocaine Self-Administration in Rats. Neuropsychopharmacology 2014, 40, 861–873. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, D.; Jang, H.B.; Chang, S.; Kim, H.K.; Ryu, Y.; Lee, B.H.; Kim, S.C.; Bills, K.B.; Steffensen, S.C.; Fan, Y.; et al. Role of Lateral Hypothalamus in Acupuncture Inhibition of Cocaine Psychomotor Activity. Int. J. Mol. Sci. 2021, 22, 5994. https://doi.org/10.3390/ijms22115994
Ahn D, Jang HB, Chang S, Kim HK, Ryu Y, Lee BH, Kim SC, Bills KB, Steffensen SC, Fan Y, et al. Role of Lateral Hypothalamus in Acupuncture Inhibition of Cocaine Psychomotor Activity. International Journal of Molecular Sciences. 2021; 22(11):5994. https://doi.org/10.3390/ijms22115994
Chicago/Turabian StyleAhn, DanBi, Han Byeol Jang, Suchan Chang, Hyung Kyu Kim, Yeonhee Ryu, Bong Hyo Lee, Sang Chan Kim, Kyle B. Bills, Scott C. Steffensen, Yu Fan, and et al. 2021. "Role of Lateral Hypothalamus in Acupuncture Inhibition of Cocaine Psychomotor Activity" International Journal of Molecular Sciences 22, no. 11: 5994. https://doi.org/10.3390/ijms22115994