Lack of WWC2 Protein Leads to Aberrant Angiogenesis in Postnatal Mice
Abstract
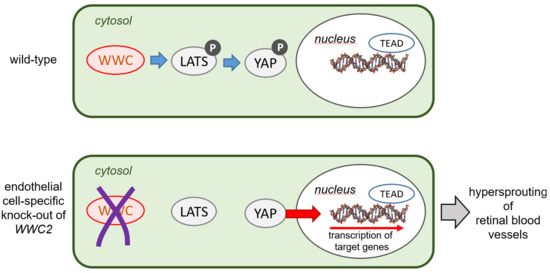
:1. Introduction
2. Results
2.1. Postnatal Animals
2.2. Adult Animals
3. Discussion
3.1. Knock-Out in Perinatal Animals
3.2. Knock-Out in Adult Animals
3.3. WWC2 Is the Decisive Factor for Vascular Development, While WWC1 Is Not Pivotal
4. Materials and Methods
4.1. Animals and Induction of the Knock-Out
4.2. In Vivo Imaging
4.3. Electroretinography
4.4. Immunohistochemical Staining of Blood Vessels
4.5. Evaluation of Retinal Whole-Mount Staining
4.6. Statistical Anazlysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, D. The hippo signaling pathway in development and cancer. Dev. Cell 2010, 19, 491–505. [Google Scholar] [CrossRef] [Green Version]
- Halder, G.; Johnson, R.L. Hippo signaling: Growth control and beyond. Development 2011, 138, 9–22. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Wang, W.; Zhang, S.; Stewart, R.; Yu, W. Identifying tumor suppressors in genetic mosaics: The Drosophila lats gene encodes a putative protein kinase. Development 1995, 121, 1053–1063. [Google Scholar] [CrossRef]
- Nguyen-Lefebvre, A.T.; Selzner, N.; Wrana, J.L.; Bhat, M. The hippo pathway: A master regulator of liver metabolism, regeneration, and disease. FASEB J. 2021, 35, e21570. [Google Scholar] [CrossRef]
- Di Benedetto, G.; Parisi, S.; Russo, T.; Passaro, F. YAP and TAZ mediators at the crossroad between metabolic and cellular reprogramming. Metabolites 2021, 11, 154. [Google Scholar] [CrossRef]
- Kremerskothen, J.; Plaas, C.; Büther, K.; Finger, I.; Veltel, S.; Matanis, T.; Liedtke, T.; Barnekow, A. Characterization of KIBRA, a novel WW domain-containing protein. Biochem. Biophys. Res. Commun. 2003, 300, 862–867. [Google Scholar] [CrossRef]
- Schneider, A.; Huentelman, M.J.; Kremerskothen, J.; Duning, K.; Spoelgen, R.; Nikolich, K. KIBRA: A new gateway to learning and memory? Front. Aging Neurosci. 2010, 2, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogt-Eisele, A.; Kruger, C.; Duning, K.; Weber, D.; Spoelgen, R.; Pitzer, C.; Plaas, C.; Eisenhardt, G.; Meyer, A.; Vogt, G.; et al. KIBRA (KIdney/BRAin protein) regulates learning and memory and stabilizes protein kinase Mζ. J. Neurochem. 2014, 128, 686–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosse, C.; Formstecher, E.; Boeckeler, K.; Zhao, Y.; Kremerskothen, J.; White, M.D.; Camonis, J.H.; Parker, P.J. An aPKC-exocyst complex controls paxillin phosphorylation and migration through localised JNK1 activation. PLoS Biol. 2009, 7, e1000235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duning, K.; Schurek, E.M.; Schlüter, M.; Bayer, M.; Reinhardt, H.C.; Schwab, A.; Schaefer, L.; Benzing, T.; Schermer, B.; Saleem, M.A.; et al. KIBRA modulates directional migration of podocytes. J. Am. Soc. Nephrol. 2008, 19, 1891–1903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mudduwa, L.; Peiris, H.; Gunasekara, S.; Abeysiriwardhana, D.; Liyanage, N.; Rayala, S.K.; Liyanage, T. KIBRA; a novel biomarker predicting recurrence free survival of breast cancer patients receiving adjuvant therapy. BMC Cancer 2018, 18, 589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knight, J.F.; Sung, V.Y.; Kuzmin, E.; Couzens, A.L.; de Verteuil, D.A.; Ratcliffe, C.D.; Coelho, P.P.; Johnson, R.M.; Samavarchi-Tehrani, P.; Gruosso, T.; et al. KIBRA (WWC1) is a metastasis suppressor gene affected by chromosome 5q loss in triple-negative breast cancer. Cell Rep. 2018, 22, 3191–3205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhou, H.; Sun, H.; Chen, J.; Huang, D.; Han, X.; Ren, X.; Lin, S.; Fan, Q.; Tian, W.; et al. Association of peripheral blood leukocyte KIBRA methylation with gastric cancer risk: A case-control study. Cancer Med. 2018, 7, 2682–2690. [Google Scholar] [CrossRef] [PubMed]
- Wennmann, D.O.; Schmitz, J.; Wehr, M.C.; Krahn, M.P.; Koschmal, N.; Gromnitza, S.; Schulze, U.; Weide, T.; Chekuri, A.; Skryabin, B.V.; et al. Evolutionary and molecular facts link the WWC protein family to Hippo signaling. Mol. Biol. Evol. 2014, 31, 1710–1723. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Ji, M.; Zhang, L.; Chen, Y.; Wennmann, D.O.; Kremerskothen, J.; Dong, J. Phosphorylation of KIBRA by the extra-cellular signal-regulated kinase (ERK)-ribosomal S6 kinase (RSK) cascade modulates cell proliferation and migration. Cell. Signal. 2014, 26, 343–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshihama, Y.; Chida, K.; Ohno, S. The KIBRA-aPKC connection: A potential regulator of membrane trafficking and cell polarity. Commun. Integr. Biol. 2012, 5, 146–151. [Google Scholar] [CrossRef] [Green Version]
- Genevet, A.; Wehr, M.C.; Brain, R.; Thompson, B.J.; Tapon, N. Kibra is a regulator of the Salvador/Warts/Hippo signaling network. Dev. Cell 2010, 18, 300–308. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Zheng, Y.; Dong, J.; Klusza, S.; Deng, W.-M.; Pan, D. Kibra functions as a tumor suppressor protein that regulates hippo signaling in conjunction with Merlin and Expanded. Dev. Cell 2010, 18, 288–299. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, R.; Poernbacher, I.; Buser, N.; Hafen, E.; Stocker, H. The WW domain protein Kibra acts upstream of hippo in drosophila. Dev. Cell 2010, 18, 309–316. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Chen, Y.; Ji, M.; Dong, J. KIBRA regulates hippo signaling activity via interactions with large tumor suppressor kinases. J. Biol. Chem. 2011, 286, 7788–7796. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Yang, S.; Wennmann, D.O.; Chen, Y.; Kremerskothen, J.; Dong, J. KIBRA: In the brain and beyond. Cell. Signal. 2014, 26, 1392–1399. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Li, X.; Huang, J.; Feng, L.; Dolinta, K.G.; Chen, J. Defining the protein-protein interaction network of the human hippo pathway. Mol. Cell. Proteom. 2014, 13, 119–131. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Deo, M.; Thompson, R.C.; Uhler, M.D.; Turner, D.L. Negative regulation of Yap during neuronal differentiation. Dev. Biol. 2012, 361, 103–115. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, R.; Lee, J.H.J.; Shin, J.; Nickas, J.; Kim, S.; Cho, S.-H. Yap is essential for retinal progenitor cell cycle progression and RPE cell fate acquisition in the developing mouse eye. Dev. Biol. 2016, 419, 336–347. [Google Scholar] [CrossRef]
- Stahl, A.; Connor, K.M.; Sapieha, P.; Chen, J.; Dennison, R.J.; Krah, N.M.; Seaward, M.R.; Willett, K.L.; Aderman, C.M.; Guerin, K.I.; et al. The mouse retina as an angiogenesis model. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2813–2826. [Google Scholar] [CrossRef]
- Selvam, S.; Kumar, T.; Fruttiger, M. Retinal vasculature development in health and disease. Prog. Retin. Eye Res. 2018, 63, 1–19. [Google Scholar] [CrossRef]
- Liu, M.; Xie, S.; Zhou, J. Use of animal models for the imaging and quantification of angiogenesis. Exp. Anim. 2018, 67, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kim, Y.H.; Kim, J.; Park, D.Y.; Bae, H.; Lee, D.-H.; Kim, K.H.; Hong, S.P.; Jang, S.P.; Kubota, Y.; et al. YAP/TAZ regulates sprouting angiogenesis and vascular barrier maturation. J. Clin. Investig. 2017, 127, 3441–3461. [Google Scholar] [CrossRef]
- Sakabe, M.; Fan, J.; Odaka, Y.; Liu, N.; Hassan, A.; Duan, X.; Stump, P.; Byerly, L.; Donaldson, M.; Hao, J.; et al. YAP/TAZ-CDC42 signaling regulates vascular tip cell migration. Proc. Natl. Acad. Sci. USA 2017, 114, 10918–10923. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.-C.; Yeh, Y.-T.; Nguyen, P.; Limqueco, E.; Lopez, J.; Thorossian, S.; Guan, K.-L.; Li, Y.-S.J.; Chien, S. Flow-dependent YAP/TAZ activities regulate endothelial phenotypes and atherosclerosis. Proc. Natl. Acad. Sci. USA 2016, 113, 11525–11530. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Luo, J.-Y.; Li, B.; Tian, X.Y.; Chen, L.-J.; Huang, Y.; Liu, J.; Deng, D.; Lau, C.W.; Wan, S.; et al. Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nat. Cell Biol. 2016, 540, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Bertero, T.; Oldham, W.M.; Cottrill, K.A.; Pisano, S.; Vanderpool, R.R.; Yu, Q.; Zhao, J.; Tai, Y.; Tang, Y.; Zhang, Y.Y.; et al. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J. Clin. Investig. 2016, 126, 3313–3335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.W.; Guan, K.-L. Regulation of the Hippo pathway and implications for anticancer drug development. Trends Pharmacol. Sci. 2013, 34, 581–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermann, A.; Wu, G.; Nedvetsky, P.I.; Brücher, V.C.; Egbring, C.; Bonse, J.; Höffken, V.; Wennmann, D.O.; Marks, M.; Krahn, M.P.; et al. The Hippo pathway component WWC2 is a key regulator of embryonic development and angiogenesis in mice. Cell. Death Dis. 2021, 12, 117. [Google Scholar] [CrossRef] [PubMed]
- Pitulescu, M.E.; Schmidt, I.; Benedito, R.; Adams, R.H. Inducible gene targeting in the neonatal vasculature and analysis of retinal angiogenesis in mice. Nat. Protoc. 2010, 5, 1518–1534. [Google Scholar] [CrossRef]
- Hellström, M.; Phng, L.-K.; Hofmann, J.J.; Wallgard, E.; Coultas, L.; Lindblom, P.; Alva, J.A.; Nilsson, A.-K.; Karlsson, L.; Gaiano, N.; et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nat. Cell Biol. 2007, 445, 776–780. [Google Scholar] [CrossRef]
- Lobov, I.B.; Renard, R.A.; Papadopoulos, N.; Gale, N.W.; Thurston, G.; Yancopoulos, G.D.; Wiegand, S.J. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc. Natl. Acad. Sci. USA 2007, 104, 3219–3224. [Google Scholar] [CrossRef] [Green Version]
- Suchting, S.; Freitas, C.; le Noble, F.; Benedito, R.; Breant, C.; Duarte, A.; Eichmann, A. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc. Natl. Acad. Sci. USA 2007, 104, 3225–3230. [Google Scholar] [CrossRef] [Green Version]
- Neto, F.; Klaus-Bergmann, A.; Ong, Y.T.; Alt, S.; Vion, A.-C.; Szymborska, A.; Carvalho, J.R.; Hollfinger, I.; Bartels-Klein, E.; Franco, C.A.; et al. YAP and TAZ regulate adherens junction dynamics and endothelial cell distribution during vascular development. eLife 2018, 7, e31037. [Google Scholar] [CrossRef]
- Zhao, B.; Ye, X.; Yu, J.; Li, L.; Li, W.; Li, S.; Lin, J.D.; Wang, C.-Y.; Chinnaiyan, A.M.; Lai, Z.-C.; et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008, 22, 1962–1971. [Google Scholar] [CrossRef] [Green Version]
- Babic, A.M.; Kireeva, M.L.; Kolesnikova, T.V.; Lau, L.F. CYR61, a product of a growth factor-inducible immediate early gene, promotes angiogenesis and tumor growth. Proc. Natl. Acad. Sci. USA 1998, 95, 6355–6360. [Google Scholar] [CrossRef] [Green Version]
- Grzeszkiewicz, T.M.; Kirschling, D.J.; Chen, N.; Lau, L.F. CYR61 stimulates human skin fibroblast migration through Integrin αvβ5 and enhances mitogenesis through integrin αvβ3, independent of its carboxyl-terminal domain. J. Biol. Chem. 2001, 276, 21943–21950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.-J.; Zhang, H.; Park, H.; Choi, K.-S.; Lee, H.W.; Agrawal, V.; Kim, Y.-M.; Kwon, Y.-G. Yes-associated protein regulates endothelial cell contact-mediated expression of angiopoietin-2. Nat. Commun. 2015, 6, 6943. [Google Scholar] [CrossRef] [PubMed]
- van der Stoel, M.; Schimmel, L.; Nawaz, K.; van Stalborch, A.-M.; de Haan, A.; Klaus-Bergmann, A.; Valent, E.T.; Koenis, D.S.; Van Nieuw Amerongen, G.P.; de Vries, C.J.; et al. DLC1 is a direct target of activated YAP/TAZ that drives collective migration and sprouting angiogenesis. J. Cell Sci. 2020, 133. [Google Scholar] [CrossRef]
- Nedvetsky, P.I.; Zhao, X.; Mathivet, T.; Aspalter, I.M.; Stanchi, F.; Metzger, R.J.; Mostov, K.E.; Gerhardt, H. cAMP-dependent protein kinase A (PKA) regulates angiogenesis by modulating tip cell behavior in a Notch-independent manner. Development 2016, 143, 3582–3590. [Google Scholar] [CrossRef] [Green Version]
- Stenzel, D.; A Franco, C.; Estrach, S.; Mettouchi, A.; Sauvaget, D.; Rosewell, I.; Schertel, A.; Armer, H.; Domogatskaya, A.; Rodin, S.; et al. Endothelial basement membrane limits tip cell formation by inducing Dll4/Notch signalling in vivo. EMBO Rep. 2011, 12, 1135–1143. [Google Scholar] [CrossRef] [Green Version]
- Dorrell, M.I.; Aguilar, E.; Friedlander, M. Retinal vascular development is mediated by endothelial filopodia, a preexisting astrocytic template and specific R-cadherin adhesion. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3500–3510. [Google Scholar]
- Murakami, M.; Simons, M. Regulation of vascular integrity. J. Mol. Med. 2009, 87, 571–582. [Google Scholar] [CrossRef] [Green Version]
- Makuch, L.; Volk, L.; Anggono, V.; Johnson, R.C.; Yu, Y.; Duning, K.; Kremerskothen, J.; Xia, J.; Takamiya, K.; Huganir, R.L. Regulation of AMPA receptor function by the human memory-associated gene KIBRA. Neuron 2011, 71, 1022–1029. [Google Scholar] [CrossRef] [Green Version]
- Hermann, A.; Wennmann, D.O.; Gromnitza, S.; Edeling, M.; Van Marck, V.; Sudol, M.; Schaefer, L.; Duning, K.; Weide, T.; Pavenstädt, H.; et al. WW and C2 domain-containing proteins regulate hepatic cell differentiation and tumor-igenesis through the hippo signaling pathway. Hepatology 2018, 67, 1546–1559. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.K.; Yang, F.; Kaul, R.; Alkan, C.; Antonellis, A.; Friery, K.F.; Zhu, B.; De Jong, P.J.; Disteche, C.M. Clcn4-2 genomic structure differs between the X locus in Mus spretus and the autosomal locus in Mus musculus: AT motif enrichment on the X. Genome Res. 2011, 21, 402–409. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Spielmann, M.; Qiu, X.; Huang, X.; Ibrahim, D.M.; Hill, A.J.; Zhang, F.; Mundlos, S.; Christiansen, L.; Steemers, F.J.; et al. The single-cell transcriptional landscape of mammalian organogenesis. Nat. Cell Biol. 2019, 566, 496–502. [Google Scholar] [CrossRef]
- Song, L.; Huang, Y.; Zhang, X.; Han, S.; Hou, M.; Li, H. Downregulation of microRNA-224-3p hampers retinoblastoma progression via activation of the hippo-YAP signaling pathway by increasing LATS2. Investig. Ophthalmol. Vis. Sci. 2020, 61, 32. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Zou, R.; Zhang, X.; Shen, M.; Chen, X.; Wang, J.; Niu, W.; Yuan, Y.; Yuan, F. YAP promotes ocular neovascularization by modifying PFKFB3-driven endothelial glycolysis. Angiogenesis 2021, 1–16. [Google Scholar] [CrossRef]
- Sörensen, I.; Adams, R.H.; Gossler, A. DLL1-mediated Notch activation regulates endothelial identity in mouse fetal arteries. Blood 2009, 113, 5680–5688. [Google Scholar] [CrossRef] [Green Version]
- Alex, A.F.; Alnawaiseh, M.; Heiduschka, P.; Eter, N. Retinal fundus imaging in mouse models of retinal diseases. Methods Mol. Biol. 2018, 1834, 253–283. [Google Scholar] [CrossRef]
- Heiduschka, P.; Schnichels, S.; Fuhrmann, N.; Hofmeister, S.; Schraermeyer, U.; Wissinger, B.; Alavi, M.V. Electrophysiological and histologic assessment of retinal ganglion cell fate in a mouse model for OPA1-associated autosomal dominant optic atrophy. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1424–1431. [Google Scholar] [CrossRef] [Green Version]
- Zudaire, E.; Gambardella, L.; Kurcz, C.; Vermeren, S. A computational tool for quantitative analysis of vascular networks. PLoS ONE 2011, 6, e27385. [Google Scholar] [CrossRef] [Green Version]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brücher, V.C.; Egbring, C.; Plagemann, T.; Nedvetsky, P.I.; Höffken, V.; Pavenstädt, H.; Eter, N.; Kremerskothen, J.; Heiduschka, P. Lack of WWC2 Protein Leads to Aberrant Angiogenesis in Postnatal Mice. Int. J. Mol. Sci. 2021, 22, 5321. https://doi.org/10.3390/ijms22105321
Brücher VC, Egbring C, Plagemann T, Nedvetsky PI, Höffken V, Pavenstädt H, Eter N, Kremerskothen J, Heiduschka P. Lack of WWC2 Protein Leads to Aberrant Angiogenesis in Postnatal Mice. International Journal of Molecular Sciences. 2021; 22(10):5321. https://doi.org/10.3390/ijms22105321
Chicago/Turabian StyleBrücher, Viktoria Constanze, Charlotte Egbring, Tanja Plagemann, Pavel I. Nedvetsky, Verena Höffken, Hermann Pavenstädt, Nicole Eter, Joachim Kremerskothen, and Peter Heiduschka. 2021. "Lack of WWC2 Protein Leads to Aberrant Angiogenesis in Postnatal Mice" International Journal of Molecular Sciences 22, no. 10: 5321. https://doi.org/10.3390/ijms22105321