Trefoil Factor Family (TFF) Modules Are Characteristic Constituents of Separate Mucin Complexes in the Xenopus laevis Integumentary Mucus: In Vitro Binding Studies with FIM-A.1
Abstract
:1. Introduction
2. Results
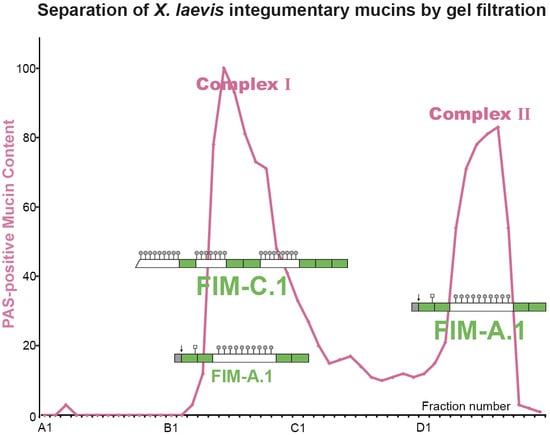
2.1. Characterization of X. laevis Skin Extracts by SEC and Western Blot Analysis
2.2. Purification of X. laevis FIM-A.1 by SEC and Anion-Exchange Chromatography
2.3. In Vitro Binding Studies of Mucus Fractions with Radioactively Labeled FIM-A.1 (Overlay Assays)
3. Discussion
3.1. The Frog Integumentary Mucus Consists of Two Complexes with Different Molecular Masses
3.2. FIM-A.1 Binds to High-Molecular-Mass Mucins of Complex I
4. Materials and Methods
4.1. Extraction of Proteins and Purification by SEC and Anion-Exchange Chromatography
4.2. SDS-PAGE, Agarose Gel Electrophoresis, and Western Blot Analysis
4.3. In Vitro Binding Studies with FIM-A.1 (Overlay Assays)
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AgGE | Agarose gel electrophoresis |
| FIM | Frog integumentary mucin |
| PAS | Periodic acid-Schiff |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide electrophoresis |
| SEC | Size-exclusion chromatography |
| TFF | Trefoil factor family |
References
- Hoffmann, W.; Bach, T.C.; Seliger, H.; Kreil, G. Biosynthesis of caerulein in the skin of Xenopus laevis: Partial sequences of precursors as deduced from cDNA clones. EMBO J. 1983, 2, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W.; Richter, K.; Kreil, G. A novel peptide designated PYLa and its precursor as predicted from cloned mRNA of Xenopus laevis skin. EMBO J. 1983, 2, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Bevins, C.L.; Zasloff, M. Peptides from frog skin. Annu. Rev. Biochem. 1990, 59, 395–414. [Google Scholar] [CrossRef] [PubMed]
- Flucher, B.E.; Lenglachner-Bachinger, C.; Pohlhammer, K.; Adam, H.; Mollay, C. Skin peptides in Xenopus laevis: Morphological requirements for precursor processing in developing and regenerating granular skin glands. J. Cell Biol. 1986, 103, 2299–2309. [Google Scholar] [CrossRef] [Green Version]
- Ramsey, J.P.; Reinert, L.K.; Harper, L.K.; Woodhams, D.C.; Rollins-Smith, L.A. Immune defenses against Batrachochytrium dendrobatidis, a fungus linked to global amphibian declines, in the South African clawed frog, Xenopus laevis. Infect. Immun. 2010, 78, 3981–3992. [Google Scholar] [CrossRef] [Green Version]
- Dubaissi, E.; Rousseau, K.; Hughes, G.W.; Ridley, C.; Grencis, R.K.; Roberts, I.S.; Thornton, D.J. Functional characterization of the mucus barrier on the Xenopus tropicalis skin surface. Proc. Natl. Acad. Sci. USA 2018, 115, 726–731. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, W.; Hauser, F. Biosynthesis of frog skin mucins: Cysteine-rich shuffled modules, polydispersities and genetic polymorphism. Comp. Biochem. Physiol. B 1993, 105, 465–472. [Google Scholar] [CrossRef]
- Lang, T.; Hansson, G.C.; Samuelsson, T. Gel-forming mucins appeared early in metazoan evolution. Proc. Natl. Acad. Sci. USA 2007, 104, 16209–16214. [Google Scholar] [CrossRef] [Green Version]
- Engelhardt, J.F.; Smith, S.S.; Allen, E.; Yankaskas, J.R.; Dawson, D.C.; Wilson, J.M. Coupled secretion of chloride and mucus in skin of Xenopus laevis: Possible role for CFTR. Am. J. Physiol. 1994, 267, C491–C500. [Google Scholar] [CrossRef]
- Perez-Vilar, J.; Boucher, R.C. Reevaluating gel-forming mucins’ roles in cystic fibrosis lung disease. Free Radic. Biol. Med. 2004, 37, 1564–1577. [Google Scholar] [CrossRef]
- Gustafsson, J.K.; Ermund, A.; Ambort, D.; Johansson, M.E.; Nilsson, H.E.; Thorell, K.; Hebert, H.; Sjovall, H.; Hansson, G.C. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J. Exp. Med. 2012, 209, 1263–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, W. A new repetitive protein from Xenopus laevis skin highly homologous to pancreatic spasmolytic polypeptide. J. Biol. Chem. 1988, 263, 7686–7690. [Google Scholar] [PubMed]
- Hauser, F.; Gertzen, E.M.; Hoffmann, W. Expression of spasmolysin (FIM-A.1): An integumentary mucin from Xenopus laevis. Exp. Cell Res. 1990, 189, 157–162. [Google Scholar] [CrossRef]
- Hoffmann, W.; Joba, W. Biosynthesis and molecular architecture of gel-forming mucins: Implications from an amphibian model system. Biochem. Soc. Trans. 1995, 23, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W.; Hauser, F. The P-domain or trefoil motif: A role in renewal and pathology of mucous epithelia? Trends Biochem. Sci. 1993, 18, 239–243. [Google Scholar] [CrossRef]
- Hoffmann, W. Trefoil factor family (TFF) peptides: Regulators of mucosal regeneration and repair, and more. Peptides 2004, 25, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Kjellev, S. The trefoil factor family – small peptides with multiple functionalities. Cell. Mol. Life Sci. 2009, 66, 1350–1369. [Google Scholar] [CrossRef]
- Hoffmann, W. TFF Peptides. In Handbook of Biologically Active Peptides, 2nd ed.; Kastin, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1338–1345. [Google Scholar]
- Braga Emidio, N.; Hoffmann, W.; Brierly, S.M.; Muttenthaler, M. Trefoil factor family: Unresolved questions and clinical perspectives. Trends Biochem. Sci. 2019, 44, 387–390. [Google Scholar] [CrossRef]
- Hauser, F.; Hoffmann, W. P-domains as shuffled cysteine-rich modules in integumentary mucin C.1 (FIM-C.1) from Xenopus laevis. Polydispersity and genetic polymorphism. J. Biol. Chem. 1992, 267, 24620–24624. [Google Scholar]
- Probst, J.C.; Gertzen, E.M.; Hoffmann, W. An integumentary mucin (FIM-B.1) from Xenopus laevis homologous with von Willebrand factor. Biochemistry 1990, 29, 6240–6244. [Google Scholar] [CrossRef]
- Probst, J.C.; Hauser, F.; Joba, W.; Hoffmann, W. The polymorphic integumentary mucin B.1 from Xenopus laevis contains the short consensus repeat. J. Biol. Chem. 1992, 267, 6310–6316. [Google Scholar] [PubMed]
- Joba, W.; Hoffmann, W. Similarities of integumentary mucin B.1 from Xenopus laevis and prepro-von Willebrand factor at their amino-terminal regions. J. Biol. Chem. 1997, 272, 1805–1810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Vilar, J.; Hill, R.L. The structure and assembly of secreted mucins. J. Biol. Chem. 1999, 274, 31751–31754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, W.; Jagla, W. Cell type specific expression of secretory TFF peptides: Colocalization with mucins and synthesis in the brain. Int. Rev. Cytol. 2002, 213, 147–181. [Google Scholar]
- Schumacher, U.; Adam, E.; Hauser, F.; Probst, J.C.; Hoffmann, W. Molecular anatomy of a skin gland: Histochemical and biochemical investigations on the mucous glands of Xenopus laevis. J. Histochem. Cytochem. 1994, 42, 57–65. [Google Scholar] [CrossRef]
- Kouznetsova, I.; Kalinski, T.; Meyer, F.; Hoffmann, W. Self-renewal of the human gastric epithelium: New insights from expression profiling using laser microdissection. Mol. Biosyst. 2011, 7, 1105–1112. [Google Scholar] [CrossRef]
- Hoffmann, W. Current status on stem cells and cancers of the gastric epithelium. Int. J. Mol. Sci. 2015, 16, 19153–19169. [Google Scholar] [CrossRef]
- Reeves, E.P.; Ali, T.; Leonard, P.; Hearty, S.; O‘Kennedy, R.; May, F.E.; Westley, B.R.; Josenhans, C.; Rust, M.; Suerbaum, S.; et al. Helicobacter pylori lipopolysaccharide interacts with TFF1 in a pH-dependent manner. Gastroenterology 2008, 135, 2043–2054. [Google Scholar] [CrossRef]
- Hoffmann, W. TFF2, a MUC6-binding lectin stabilizing the gastric mucus barrier and more. Int. J. Oncol. 2015, 47, 806–816. [Google Scholar] [CrossRef] [Green Version]
- Stürmer, R.; Reising, J.; Hoffmann, W. The TFF Peptides xP1 and xP4 Appear in Distinctive Forms in the Xenopus laevis Gastric Mucosa: Indications for Different Protective Functions. Int. J. Mol. Sci. 2019, 20, 6052. [Google Scholar] [CrossRef] [Green Version]
- Heuer, F.; Stürmer, R.; Heuer, J.; Kalinski, T.; Lemke, A.; Meyer, F.; Hoffmann, W. Different forms of TFF2, a lectin of the human gastric mucus barrier: In vitro binding studies. Int. J. Mol. Sci. 2019, 20, 5871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albert, T.K.; Laubinger, W.; Müller, S.; Hanisch, F.-G.; Kalinski, T.; Meyer, f.; Hoffmann, W. Human intestinal TFF3 forms disulfide-linked heteromers with the mucus-associated FCGBP protein and is released by hydrogen sulfide. J. Proteome Res. 2010, 9, 3108–3117. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.V.; Sjövall, H.; Hansson, G.C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 352–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauser, F.; Roeben, C.; Hoffmann, W. xP2, a new member of the P-domain peptide family of potential growth factors, is synthesized in Xenopus laevis skin. J. Biol. Chem. 1992, 267, 14451–14455. [Google Scholar]
- Liu, S.-B.; He, Y.-Y.; Zhang, Y.; Lee, W.-H.; Qian, J.-Q.; Lai, R.; Jin, Y. A novel non-lens βγ-crystallin and trefoil factor complex from amphibian skin and its functional implications. PLoS ONE 2008, 3, 1770. [Google Scholar] [CrossRef] [Green Version]
- Marschal, P.; Herrmann, J.; Leffler, H.; Barondes, S.H.; Cooper, D.N.W. Sequence and specificity of a soluble lectin from Xenopus laevis skin. J. Biol. Chem. 1992, 267, 12942–12949. [Google Scholar]
- Shoji, H.; Nishi, N.; Hirashima, M.; Nakamura, T. Characterization of Xenopus galectin family. J. Biol. Chem. 2003, 278, 12285–12293. [Google Scholar] [CrossRef] [Green Version]
- Hanisch, F.-G.; Ragge, H.; Kalinski, T.; Meyer, F.; Kalbacher, H.; Hoffmann, W. Human gastric TFF2 peptide contains an N-linked fucosylated N,N′-diacetyllactosediamine (LacdiNAc) oligosaccharide. Glycobiology 2013, 23, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Stürmer, R.; Müller, S.; Hanisch, F.-G.; Hoffmann, W. Porcine gastric TFF2 is a mucus constituent and differs from pancreatic TFF2. Cell. Physiol. Biochem. 2014, 33, 895–904. [Google Scholar] [CrossRef]
- Stürmer, R.; Harder, S.; Schlüter, H.; Hoffmann, W. Commercial porcine gastric mucin preparations, also used as artificial saliva, are a rich source for the lectin TFF2: In vitro binding studies. ChemBioChem 2018, 19, 2598–2608. [Google Scholar] [CrossRef]
- Thornton, D.J.; Holmes, D.F.; Sheehan, J.K.; Carlstedt, I. Quantitation of mucus glycoproteins blotted onto nitrocellulose membranes. Anal. Biochem. 1989, 182, 160–164. [Google Scholar] [CrossRef]
- Blum, H.; Beier, H.; Gross, H.J. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 1987, 8, 93–99. [Google Scholar] [CrossRef]
- Hauser, F. Molekulare Analyse Neuer P-Domänen: Cysteinreiche Module in Sekretorischen Peptiden und Muzinen. Ph.D. Thesis, Ludwig-Maximilian-University, Munich, Germany, 1992. [Google Scholar]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stürmer, R.; Reising, J.; Hoffmann, W. Trefoil Factor Family (TFF) Modules Are Characteristic Constituents of Separate Mucin Complexes in the Xenopus laevis Integumentary Mucus: In Vitro Binding Studies with FIM-A.1. Int. J. Mol. Sci. 2020, 21, 2400. https://doi.org/10.3390/ijms21072400
Stürmer R, Reising J, Hoffmann W. Trefoil Factor Family (TFF) Modules Are Characteristic Constituents of Separate Mucin Complexes in the Xenopus laevis Integumentary Mucus: In Vitro Binding Studies with FIM-A.1. International Journal of Molecular Sciences. 2020; 21(7):2400. https://doi.org/10.3390/ijms21072400
Chicago/Turabian StyleStürmer, René, Jana Reising, and Werner Hoffmann. 2020. "Trefoil Factor Family (TFF) Modules Are Characteristic Constituents of Separate Mucin Complexes in the Xenopus laevis Integumentary Mucus: In Vitro Binding Studies with FIM-A.1" International Journal of Molecular Sciences 21, no. 7: 2400. https://doi.org/10.3390/ijms21072400