Long-Acting FGF21 Inhibits Retinal Vascular Leakage in In Vivo and In Vitro Models
Abstract
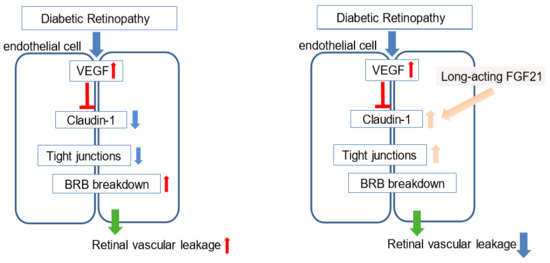
:1. Introduction
2. Results
2.1. Establishing a Machine Learning Program for Vascular Leakage Quantification
2.2. FGF21 Agonist Inhibits and FGF21 Deficiency Increases VEGF-Induced Retinal Vascular Leakage in Mice
2.3. FGF21 Decreases HRMECs Permeability in Vitro
2.4. FGF21 Stabilizes TEER in HRMECs in Vitro
2.5. FGF21 Preserves Expression of Tight Junction (Claudin-1) Protein in HRMECs
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Animal Model
4.3. Imaging and Machine Learning Analysis
4.4. Endothelial Monolayer Permeability Assay
4.5. Trans-Endothelial Electrical Resistance Assay
4.6. Western Blotting
4.7. Real-Time PCR
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DR | Diabetic retinopathy |
| FGF21 | Fibroblast growth factor 21 |
| FGFR | Fibroblast growth factor receptor |
| HRMECs | Primary human retinal microvascular endothelial cells |
| PPARα | peroxisome proliferator-activated receptor alpha |
| TEER | Trans-endothelial electrical resistance |
| VEGF | Vascular endothelial growth factor |
References
- Hendrick, A.M.; Gibson, M.V.; Kulshreshtha, A. Diabetic Retinopathy. Prim Care 2015, 42, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Ishida, S.; Usui, T.; Yamashiro, K.; Kaji, Y.; Ahmed, E.; Carrasquillo, K.G.; Amano, S.; Hida, T.; Oguchi, Y.; Adamis, A.P. VEGF164 is proinflammatory in the diabetic retina. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2155–2162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funatsu, H.; Noma, H.; Mimura, T.; Eguchi, S.; Hori, S. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology 2009, 116, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Fintak, D.R.; Shah, G.K.; Blinder, K.J.; Regillo, C.D.; Pollack, J.; Heier, J.S.; Hollands, H.; Sharma, S. Incidence of endophthalmitis related to intravitreal injection of bevacizumab and ranibizumab. Retina 2008, 28, 1395–1399. [Google Scholar] [CrossRef]
- Hernandez-Pastor, L.J.; Ortega, A.; Garcia-Layana, A.; Giraldez, J. Ranibizumab for neovascular age-related macular degeneration. Am. J. Health Syst. Pharm. 2008, 65, 1805–1814. [Google Scholar] [CrossRef]
- Pearson, P.A.; Comstock, T.L.; Ip, M.; Callanan, D.; Morse, L.S.; Ashton, P.; Levy, B.; Mann, E.S.; Eliott, D. Fluocinolone acetonide intravitreal implant for diabetic macular edema: A 3-year multicenter, randomized, controlled clinical trial. Ophthalmology 2011, 118, 1580–1587. [Google Scholar] [CrossRef]
- Keech, A.C.; Mitchell, P.; Summanen, P.A.; O’Day, J.; Davis, T.M.; Moffitt, M.S.; Taskinen, M.R.; Simes, R.J.; Tse, D.; Williamson, E.; et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): A randomised controlled trial. Lancet 2007, 370, 1687–1697. [Google Scholar] [CrossRef]
- Group, A.S.; Group, A.E.S.; Chew, E.Y.; Ambrosius, W.T.; Davis, M.D.; Danis, R.P.; Gangaputra, S.; Greven, C.M.; Hubbard, L.; Esser, B.A.; et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N. Engl. J. Med. 2010, 363, 233–244. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Hu, Y.; Lin, M.; Jenkins, A.J.; Keech, A.C.; Mott, R.; Lyons, T.J.; Ma, J.X. Therapeutic effects of PPARalpha agonists on diabetic retinopathy in type 1 diabetes models. Diabetes 2013, 62, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Noonan, J.E.; Jenkins, A.J.; Ma, J.X.; Keech, A.C.; Wang, J.J.; Lamoureux, E.L. An update on the molecular actions of fenofibrate and its clinical effects on diabetic retinopathy and other microvascular end points in patients with diabetes. Diabetes 2013, 62, 3968–3975. [Google Scholar] [CrossRef] [Green Version]
- Araki, M.; Nakagawa, Y.; Oishi, A.; Han, S.I.; Wang, Y.; Kumagai, K.; Ohno, H.; Mizunoe, Y.; Iwasaki, H.; Sekiya, M.; et al. The Peroxisome Proliferator-Activated Receptor alpha (PPARalpha) Agonist Pemafibrate Protects against Diet-Induced Obesity in Mice. Int. J. Mol. Sci. 2018, 19, 2148. [Google Scholar] [CrossRef] [Green Version]
- Tomita, Y.; Ozawa, N.; Miwa, Y.; Ishida, A.; Ohta, M.; Tsubota, K.; Kurihara, T. Pemafibrate Prevents Retinal Pathological Neovascularization by Increasing FGF21 Level in a Murine Oxygen-Induced Retinopathy Model. Int. J. Mol. Sci. 2019, 20, 5878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimura, T.; Nakatake, Y.; Konishi, M.; Itoh, N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim. Et Biophys. Acta 2000, 1492, 203–206. [Google Scholar] [CrossRef]
- Staiger, H.; Keuper, M.; Berti, L.; Hrabe de Angelis, M.; Haring, H.U. Fibroblast Growth Factor 21-Metabolic Role in Mice and Men. Endocr. Rev. 2017, 38, 468–488. [Google Scholar] [CrossRef]
- Talukdar, S.; Zhou, Y.; Li, D.; Rossulek, M.; Dong, J.; Somayaji, V.; Weng, Y.; Clark, R.; Lanba, A.; Owen, B.M.; et al. A Long-Acting FGF21 Molecule, PF-05231023, Decreases Body Weight and Improves Lipid Profile in Non-human Primates and Type 2 Diabetic Subjects. Cell Metab. 2016, 23, 427–440. [Google Scholar] [CrossRef] [Green Version]
- Fu, Z.; Gong, Y.; Liegl, R.; Wang, Z.; Liu, C.H.; Meng, S.S.; Burnim, S.B.; Saba, N.J.; Fredrick, T.W.; Morss, P.C.; et al. FGF21 Administration Suppresses Retinal and Choroidal Neovascularization in Mice. Cell Rep. 2017, 18, 1606–1613. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, Z.; Liu, C.H.; Gong, Y.; Cakir, B.; Liegl, R.; Sun, Y.; Meng, S.S.; Burnim, S.B.; Arellano, I.; et al. Fibroblast Growth Factor 21 Protects Photoreceptor Function in Type 1 Diabetic Mice. Diabetes 2018, 67, 974–985. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.Z.; Le, Y.Z. Significance of outer blood-retina barrier breakdown in diabetes and ischemia. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2160–2164. [Google Scholar] [CrossRef]
- Scheppke, L.; Aguilar, E.; Gariano, R.F.; Jacobson, R.; Hood, J.; Doukas, J.; Cao, J.; Noronha, G.; Yee, S.; Weis, S.; et al. Retinal vascular permeability suppression by topical application of a novel VEGFR2/Src kinase inhibitor in mice and rabbits. J. Clin. Investig. 2008, 118, 2337–2346. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.J.; Jung, S.H.; Kim, S.H.; Kim, M.S.; Lee, S.; Hwang, J.; Kim, S.Y.; Kim, Y.M.; Ha, K.S. Essential Role of Transglutaminase 2 in Vascular Endothelial Growth Factor-Induced Vascular Leakage in the Retina of Diabetic Mice. Diabetes 2016, 65, 2414–2428. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, N.; Tan, H.Y.; Zhang, Y.; Feng, Y. Protective effect of a Chinese Medicine formula He-Ying-Qing-Re Formula on diabetic retinopathy. J. Ethnopharmacol. 2015, 169, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Qaum, T.; Xu, Q.; Joussen, A.M.; Clemens, M.W.; Qin, W.; Miyamoto, K.; Hassessian, H.; Wiegand, S.J.; Rudge, J.; Yancopoulos, G.D.; et al. VEGF-initiated blood-retinal barrier breakdown in early diabetes. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2408–2413. [Google Scholar]
- Xiao, S.; Bucher, F.; Wu, Y.; Rokem, A.; Lee, C.S.; Marra, K.V.; Fallon, R.; Diaz-Aguilar, S.; Aguilar, E.; Friedlander, M.; et al. Fully automated, deep learning segmentation of oxygen-induced retinopathy images. JCI Insight 2017, 2. [Google Scholar] [CrossRef] [Green Version]
- Daruich, A.; Matet, A.; Moulin, A.; Kowalczuk, L.; Nicolas, M.; Sellam, A.; Rothschild, P.R.; Omri, S.; Gelize, E.; Jonet, L.; et al. Mechanisms of macular edema: Beyond the surface. Prog. Retin. Eye Res. 2018, 63, 20–68. [Google Scholar] [CrossRef] [PubMed]
- Ouban, A. Claudin-1 role in colon cancer: An update and a review. Histol. Histopathol. 2018, 33, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Xiao, W.; Zhu, X.; Mao, Y.; Liu, X.; Chen, X.; Huang, J.; Tang, S.; Rizzolo, L.J. Differential expression of claudins in retinas during normal development and the angiogenesis of oxygen-induced retinopathy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7556–7564. [Google Scholar] [CrossRef] [Green Version]
- Klaassen, I.; Hughes, J.M.; Vogels, I.M.; Schalkwijk, C.G.; Van Noorden, C.J.; Schlingemann, R.O. Altered expression of genes related to blood-retina barrier disruption in streptozotocin-induced diabetes. Exp. Eye Res. 2009, 89, 4–15. [Google Scholar] [CrossRef]
- Suarez, S.; McCollum, G.W.; Bretz, C.A.; Yang, R.; Capozzi, M.E.; Penn, J.S. Modulation of VEGF-induced retinal vascular permeability by peroxisome proliferator-activated receptor-beta/delta. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8232–8240. [Google Scholar] [CrossRef] [Green Version]
- Moyers, J.S.; Shiyanova, T.L.; Mehrbod, F.; Dunbar, J.D.; Noblitt, T.W.; Otto, K.A.; Reifel-Miller, A.; Kharitonenkov, A. Molecular determinants of FGF-21 activity-synergy and cross-talk with PPARgamma signaling. J. Cell Physiol. 2007, 210, 1–6. [Google Scholar] [CrossRef]
- Ding, X.; Boney-Montoya, J.; Owen, B.M.; Bookout, A.L.; Coate, K.C.; Mangelsdorf, D.J.; Kliewer, S.A. betaKlotho is required for fibroblast growth factor 21 effects on growth and metabolism. Cell Metab. 2012, 16, 387–393. [Google Scholar] [CrossRef] [Green Version]
- Rakoczy, E.P.; Ali Rahman, I.S.; Binz, N.; Li, C.R.; Vagaja, N.N.; de Pinho, M.; Lai, C.M. Characterization of a mouse model of hyperglycemia and retinal neovascularization. Am. J. Pathol. 2010, 177, 2659–2670. [Google Scholar] [CrossRef] [PubMed]
- Kokona, D.; Ebneter, A.; Escher, P.; Zinkernagel, M.S. Colony-stimulating factor 1 receptor inhibition prevents disruption of the blood-retina barrier during chronic inflammation. J. Neuroinflamm. 2018, 15, 340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usui, Y.; Westenskow, P.D.; Kurihara, T.; Aguilar, E.; Sakimoto, S.; Paris, L.P.; Wittgrove, C.; Feitelberg, D.; Friedlander, M.S.; Moreno, S.K.; et al. Neurovascular crosstalk between interneurons and capillaries is required for vision. J. Clin. Investig. 2015, 125, 2335–2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catalani, E.; Tomassini, S.; Dal Monte, M.; Bosco, L.; Casini, G. Localization patterns of fibroblast growth factor 1 and its receptors FGFR1 and FGFR2 in postnatal mouse retina. Cell Tissue Res. 2009, 336, 423–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinkl, N.; Hageman, G.S.; Sahel, J.A.; Hicks, D. Fibroblast growth factor receptor (FGFR) and candidate signaling molecule distribution within rat and human retina. Mol. Vis. 2002, 8, 149–160. [Google Scholar] [PubMed]
- Adachi, T.; Teramachi, M.; Yasuda, H.; Kamiya, T.; Hara, H. Contribution of p38 MAPK, NF-kappaB and glucocorticoid signaling pathways to ER stress-induced increase in retinal endothelial permeability. Arch. Biochem. Biophys. 2012, 520, 30–35. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomita, Y.; Fu, Z.; Wang, Z.; Cakir, B.; Cho, S.S.; Britton, W.; Sun, Y.; Hellström, A.; Talukdar, S.; Smith, L.E.H. Long-Acting FGF21 Inhibits Retinal Vascular Leakage in In Vivo and In Vitro Models. Int. J. Mol. Sci. 2020, 21, 1188. https://doi.org/10.3390/ijms21041188
Tomita Y, Fu Z, Wang Z, Cakir B, Cho SS, Britton W, Sun Y, Hellström A, Talukdar S, Smith LEH. Long-Acting FGF21 Inhibits Retinal Vascular Leakage in In Vivo and In Vitro Models. International Journal of Molecular Sciences. 2020; 21(4):1188. https://doi.org/10.3390/ijms21041188
Chicago/Turabian StyleTomita, Yohei, Zhongjie Fu, Zhongxiao Wang, Bertan Cakir, Steve S. Cho, William Britton, Ye Sun, Ann Hellström, Saswata Talukdar, and Lois E.H. Smith. 2020. "Long-Acting FGF21 Inhibits Retinal Vascular Leakage in In Vivo and In Vitro Models" International Journal of Molecular Sciences 21, no. 4: 1188. https://doi.org/10.3390/ijms21041188