Hybrid Porous Microparticles Based on a Single Organosilica Cyclophosphazene Precursor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Characterization Methods
2.3. Synthesis of Silane Derived Cyclic Phosphazenes SiCPz1 and SiCPz2
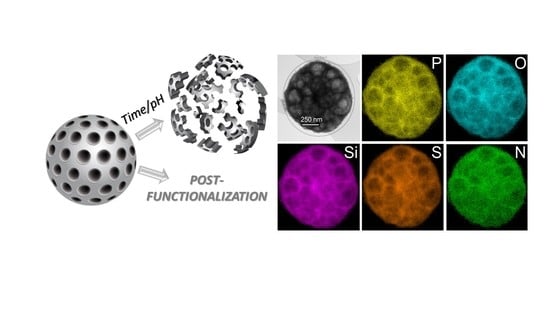
2.4. Synthesis of Porous Organosilica Phosphazene Based Microparticles SiCPz1-PM1 and SiCPz2-PM2
2.5. Hydrolytic Degradation Studies of the Porous SiCPz1-PM1 Microparticles
2.6. Post-Functionalization of the Porous SiCPz2-PM2 Microparticles
3. Results and Discussion
3.1. Synthesis and Characterization of the Cyclic Organosilica Phosphazenes Bridges
3.2. Synthesis of Porous Organosilica Phosphazene-Based Microparticles
3.3. Characterization of the Prepared Porous Organosilica Microparticles
3.4. Hydrolytic Degradation Studies of SiCPz1-PM1 Microparticles
3.5. Post-Functionalization of the Porous SiCPz2-PM2 Microparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ciriminna, R.; Fidalgo, A.; Pandarus, V.; Béland, F.; Ilharco, L.M.; Pagliaro, M. The sol-gel route to advanced silica-based materials and recent applications. Chem. Rev. 2013, 113, 6592–6620. [Google Scholar] [CrossRef] [PubMed]
- Möller, K.; Bein, T. Degradable drug carriers: Vanishing mesoporous silica nanoparticles. Chem. Mater. 2019, 31, 4364–4378. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Hu, Y.; Sun, L.; Bai, L.; Jiang, T.; Wang, S. Ordered nanoporous silica as carriers for improved delivery of water insoluble drugs: A comparative study between three dimensional and two dimensional macroporous silica. Int. J. Nanomed. 2013, 8, 4015–4031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: Boston, MA, USA, 1990. [Google Scholar]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Lai, C.Y. Mesoporous silica nanomaterials applications in catalysis. J. Thermodyn. Catal. 2013, 5, 10–12. [Google Scholar]
- Manzano, M.; Vallet-Regí, M. Mesoporous silica nanoparticles for drug delivery. Adv. Funct. Mater. 2020, 30, 3–5. [Google Scholar] [CrossRef]
- Asefa, T.; MacLachlan, M.J.; Coombs, N.; Ozin, G.A. Periodic mesoporous organosilicas with organic groups inside the channel walls. Nature 1999, 402, 867–871. [Google Scholar] [CrossRef]
- Inagaki, S.; Guan, S.; Fukushima, Y.; Ohsuna, T.; Terasaki, O. Novel mesoporous materials with a uniform distribution of organic groups and inorganic oxide in their frameworks. J. Am. Chem. Soc. 1999, 121, 9611–9614. [Google Scholar] [CrossRef]
- Hatton, B.; Landskron, K.; Whitnall, W.; Perovic, D.; Ozin, G.A. Past, present, and future of periodic mesoporous organosilicas—The PMOs. Acc. Chem. Res. 2005, 38, 305–312. [Google Scholar] [CrossRef]
- Poscher, V.; Salinas, Y. Trends in degradable mesoporous organosilica-based nanomaterials for controlling drug delivery: A mini review. Materials 2020, 13, 3668. [Google Scholar] [CrossRef] [PubMed]
- Rothemund, S.; Teasdale, I. Preparation of polyphosphazenes: A tutorial review. Chem. Soc. Rev. 2016, 45, 5200–5215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strasser, P.; Teasdale, I. Main-chain phosphorus-containing polymers for therapeutic applications. Molecules 2020, 25, 1716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teasdale, I.; Brüggemann, O.; Henke, H. Polyphosphazenes for Medical Applications; De Gruyter: Berlin, NH, USA, 2020. [Google Scholar]
- Allcock, H.R.; Morozowich, N.L. Bioerodible polyphosphazenes and their medical potential. Polym. Chem. 2012, 3, 578–590. [Google Scholar] [CrossRef]
- Caminade, A.M.; Hameau, A.; Majoral, J.P. The specific functionalization of cyclotriphosphazene for the synthesis of smart dendrimers. Dalton Trans. 2016, 45, 1810–1822. [Google Scholar] [CrossRef]
- Xiong, S.; Tao, J.; Wang, Y.; Tang, J.; Liu, C.; Liu, Q.; Wang, Y.; Yu, G.; Pan, C. Uniform poly(phosphazene-triazine) porous microspheres for highly efficient iodine removal. Chem. Commun. 2018, 54, 8450–8453. [Google Scholar] [CrossRef]
- Wang, S.; Sui, X.; Li, Y.; Li, J.; Xu, H.; Zhong, Y.; Zhang, L.; Mao, Z. Durable flame retardant finishing of cotton fabrics with organosilicon functionalized cyclotriphosphazene. Polym. Degrad. Stab. 2016, 128, 22–28. [Google Scholar] [CrossRef]
- Christova, D.; Ivanova, S.D.; Velichkova, R.S.; Tzvetkova, P.; Mihailova, P.; Lakov, L.; Peshev, O. New functionalized cyclotriphosphazenes-synthesis and application in the sol-gel process. Des. Monomers Polym. 2001, 4, 329–341. [Google Scholar] [CrossRef] [Green Version]
- Khanin, D.A.; Kononevich, Y.N.; Temnikov, M.N.; Morgalyuk, V.P.; Vasil’ev, V.G.; Popov, A.Y.; Brel, V.K.; Muzafarov, A.M. New hybrid materials based on cyclophosphazene and polysiloxane precursors: Synthesis and properties. Polymer 2020, 186, 122011. [Google Scholar] [CrossRef]
- Jiménez, J.; Laguna, A.; Gascón, E.; Sanz, J.A.; Serrano, J.L.; Barberá, J.; Oriol, L. New liquid crystalline materials based on two generations of dendronised cyclophosphazenes. Chem. Eur. J. 2012, 18, 16801–16814. [Google Scholar]
- Jiang, J.; Wang, Y.; Luo, Z.; Qi, T.; Qiao, Y.; Zuo, M.; Wang, B. Design and application of highly efficient flame retardants for polycarbonate combining the advantages of cyclotriphosphazene and silicone oil. Polymers 2019, 11, 1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poscher, V.; Teasdale, I.; Salinas, Y. Surfactant-free synthesis of cyclomatrix and linear organosilica phosphazene-based hybrid nanoparticles. ACS Appl. Nano Mater. 2019, 2, 655–660. [Google Scholar] [CrossRef]
- Qian, Y.-C.; Huang, X.-J.; Chen, C.; Ren, N.; Huang, X.; Xu, Z.-K. A versatile approach to the synthesis of polyphosphazene derivatives via the thiol-ene reaction. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 5170–5176. [Google Scholar] [CrossRef]
- Wang, D.; Feng, X.; Zhang, L.; Li, M.; Liu, M.; Tian, A.; Fu, S. Cyclotriphosphazene-bridged periodic mesoporous organosilica-integrated cellulose nanofiber anisotropic foam with highly flame-retardant and thermally insulating properties. Chem. Eng. J. 2019, 375, 121933. [Google Scholar] [CrossRef]
- Henke, H.; Wilfert, S.; Iturmendi, A.; Brüggemann, O.; Teasdale, I. Branched polyphosphazenes with controlled dimensions. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 4467–4473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croissant, J.G.; Mauriello-Jimenez, C.; Maynadier, M.; Cattoën, X.; Wong Chi Man, M.; Raehm, L.; Mongin, O.; Blanchard-Desce, M.; Garcia, M.; Gary-Bobo, M.; et al. Synthesis of disulfide-based biodegradable bridged silsesquioxane nanoparticles for two-photon imaging and therapy of cancer cells. Chem. Commun. 2015, 51, 12324–12327. [Google Scholar] [CrossRef]
- Wilfert, S.; Iturmendi, A.; Schoefberger, W.; Kryeziu, K.; Heffeter, P.; Berger, W.; Brüggemann, O.; Teasdale, I. Water-soluble, biocompatible polyphosphazenes with controllable and pH-promoted degradation behavior. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, M.C.; Aggad, D.; Croissant, J.G.; Tresfield, K.; Laurencin, D.; Berthomieu, D.; Cubedo, N.; Rossel, M.; Alsaiari, S.; Anjum, D.H.; et al. Porous porphyrin-based organosilica nanoparticles for nir two-photon photodynamic therapy and gene delivery in Zebrafish. Adv. Funct. Mater. 2018, 28, 1800235. [Google Scholar]
- Barczak, M.; Dobrowolski, R.; Borowski, P.; Giannakoudakis, D.A. Pyridine-, thiol- and amine-functionalized mesoporous silicas for adsorptive removal of pharmaceuticals. Microporous Mesoporous Mater. 2020, 299, 110132. [Google Scholar] [CrossRef]
- Mangos, D.N.; Nakanishi, T.; Lewis, D.A. A simple method for the quantification of molecular decorations on silica particles. Sci. Technol. Adv. Mater. 2014, 15, 015002. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poscher, V.; Pappas, G.S.; Brüggemann, O.; Teasdale, I.; Salinas, Y. Hybrid Porous Microparticles Based on a Single Organosilica Cyclophosphazene Precursor. Int. J. Mol. Sci. 2020, 21, 8552. https://doi.org/10.3390/ijms21228552
Poscher V, Pappas GS, Brüggemann O, Teasdale I, Salinas Y. Hybrid Porous Microparticles Based on a Single Organosilica Cyclophosphazene Precursor. International Journal of Molecular Sciences. 2020; 21(22):8552. https://doi.org/10.3390/ijms21228552
Chicago/Turabian StylePoscher, Vanessa, George S. Pappas, Oliver Brüggemann, Ian Teasdale, and Yolanda Salinas. 2020. "Hybrid Porous Microparticles Based on a Single Organosilica Cyclophosphazene Precursor" International Journal of Molecular Sciences 21, no. 22: 8552. https://doi.org/10.3390/ijms21228552