An Analysis of Clinical, Surgical, Pathological and Molecular Characteristics of Endometrial Cancer According to Mismatch Repair Status. A Multidisciplinary Approach
Abstract
:1. Introduction
2. Results
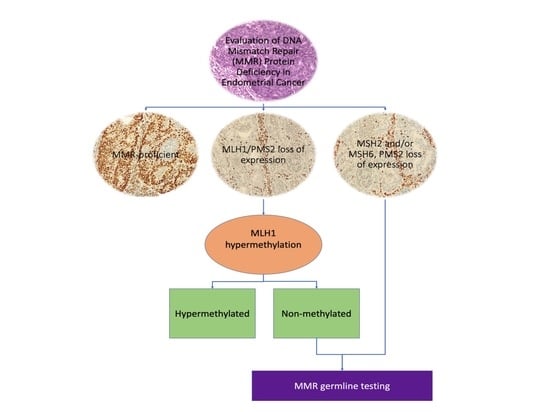
2.1. IHC Analysis and MMR Protein Expression Assessment
2.2. Germline MMR Status Assessment and LS Diagnosis
2.3. Correlation Between Clinical and Pathological Features and Germline Variants in Patients with LS
2.4. Comparison Among the Three Groups (LS, LLC, MMR-Proficient) for
2.4.1. Personal and Familial Cancer History
2.4.2. Clinical Features
2.4.3. Surgical Treatments
2.4.4. Pathological Analysis
2.4.5. Postoperative Treatment and Outcome
2.4.6. Effects of MMR Status on Clinical and Pathological Variables
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Data Collection
4.3. Surgical Protocol
4.4. IHC-Screening
4.5. MLH1 Promoter Methylation
4.6. MSI Molecular Testing
4.7. Genetic Counseling and Germline Testing
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Raglan, O.; Kalliala, I.; Markozannes, G.; Cividini, S.; Gunter, M.J.; Nautiyal, J.; Gabra, H.; Paraskevaidis, E.; Martin-Hirsch, P.; Tsilidis, K.K.; et al. Risk factors for endometrial cancer: An umbrella review of the literature: Risk factors for endometrial cancer. Int. J. Cancer 2019, 145, 1719–1730. [Google Scholar] [CrossRef] [Green Version]
- Levine, D.A. The Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Li-Chang, H.H.; Kwon, J.S.; Melnyk, N.; Yang, W.; Senz, J.; Boyd, N.; Karnezis, A.N.; et al. A clinically applicable molecular-based classification for endometrial cancers. Br. J. Cancer 2015, 113, 299–310. [Google Scholar] [CrossRef] [Green Version]
- Kommoss, S.; McConechy, M.K.; Kommoss, F.; Leung, S.; Bunz, A.; Magrill, J.; Britton, H.; Kommoss, F.; Grevenkamp, F.; Karnezis, A.; et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Ann. Oncol. 2018, 29, 1180–1188. [Google Scholar] [CrossRef]
- Yamamoto, H.; Imai, K. Microsatellite instability: An update. Arch. Toxicol. 2015, 89, 899–921. [Google Scholar] [CrossRef]
- Ryan, N.A.J.; Glaire, M.A.; Blake, D.; Cabrera-Dandy, M.; Evans, D.G.; Crosbie, E.J. The proportion of endometrial cancers associated with Lynch syndrome: A systematic review of the literature and meta-analysis. Genet. Med. 2019, 21, 2167–2180. [Google Scholar] [CrossRef] [Green Version]
- Møller, P.; Seppälä, T.; Bernstein, I.; Holinski-Feder, E.; Sala, P.; Evans, D.G.; Lindblom, A.; Macrae, F.; Blanco, I.; Sijmons, R.; et al. Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: First report from the prospective Lynch syndrome database. Gut 2017, 66, 464–472. [Google Scholar] [CrossRef]
- Lynch, H.T.; Snyder, C.L.; Shaw, T.G.; Heinen, C.D.; Hitchins, M.P. Milestones of Lynch syndrome: 1895–2015. Nat. Rev. Cancer 2015, 15, 181–194. [Google Scholar] [CrossRef]
- Hampel, H.; Frankel, W.L.; Martin, E.; Arnold, M.; Khanduja, K.; Kuebler, P.; Clendenning, M.; Sotamaa, K.; Prior, T.; Westman, J.A.; et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 5783–5788. [Google Scholar] [CrossRef]
- Lu, K.H.; Dinh, M.; Kohlmann, W.; Watson, P.; Green, J.; Syngal, S.; Bandipalliam, P.; Chen, L.-M.; Allen, B.; Conrad, P.; et al. Gynecologic cancer as a “Sentinel Cancer” for women with hereditary nonpolyposis colorectal cancer syndrome. Obstet. Gynecol. 2005, 105, 569–574. [Google Scholar] [CrossRef]
- Clarke, B.A.; Cooper, K. Identifying Lynch syndrome in patients with endometrial carcinoma: Shortcomings of morphologic and clinical schemas. Adv. Anat. Pathol. 2012, 19, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Heald, B.; Plesec, T.; Liu, X.; Pai, R.; Patil, D.; Moline, J.; Sharp, R.R.; Burke, C.A.; Kalady, M.F.; Church, J.; et al. Implementation of universal microsatellite instability and immunohistochemistry screening for diagnosing Lynch syndrome in a large academic medical center. J. Clin. Oncol. 2013, 31, 1336–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giardiello, F.M.; Allen, J.I.; Axilbund, J.E.; Boland, C.R.; Burke, C.A.; Burt, R.W.; Church, J.M.; Dominitz, J.A.; Johnson, D.A.; Kaltenbach, T.; et al. Guidelines on genetic evaluation and management of Lynch syndrome: A consensus statement by the us multi-society task force on colorectal cancer. Gastroenterology 2014, 147, 502–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Provenzale, D.; Gupta, S.; Ahnen, D.J.; Bray, T.; Cannon, J.A.; Cooper, G.; David, D.S.; Early, D.S.; Erwin, D.; Ford, J.M.; et al. Genetic/Familial high-risk assessment: Colorectal version 1.2016, nccn clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 2016, 14, 1010–1030. [Google Scholar] [CrossRef]
- Chen, L.M.; Cohn, D.E.; Fishman, D.A.; Gibb, R.K.; Mutch, D.G.; Olawaiye, A.B.; Soper, D.E. Practice Bulletin No. 147: Lynch Syndrome. Obstet. Gynecol. 2014, 124, 1042–1054. [Google Scholar] [CrossRef]
- Ryan, P.; Mulligan, A.M.; Aronson, M.; Ferguson, S.E.; Bapat, B.; Semotiuk, K.; Holter, S.; Kwon, J.; Kalloger, S.E.; Gilks, C.B.; et al. Comparison of clinical schemas and morphologic features in predicting Lynch syndrome in mutation-positive patients with endometrial cancer encountered in the context of familial gastrointestinal cancer registries. Cancer 2012, 118, 681–688. [Google Scholar] [CrossRef]
- Hampel, H.; Frankel, W.; Panescu, J.; Lockman, J.; Sotamaa, K.; Fix, D.; Comeras, I.; La Jeunesse, J.; Nakagawa, H.; Westman, J.A.; et al. Screening for Lynch Syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res. 2006, 66, 7810–7817. [Google Scholar] [CrossRef] [Green Version]
- Syngal, S.; Fox, E.A.; Eng, C.; Kolodner, R.D.; Garber, J.E. Sensitivity and specificity of clinical criteria for hereditary non-polyposis colorectal cancer associated mutations in MSH2 and MLH. J. Med. Genet. 2000, 37, 641–645. [Google Scholar] [CrossRef] [Green Version]
- Resnick, K.E.; Hampel, H.; Fishel, R.; Cohn, D.E. Current and emerging trends in Lynch syndrome identification in women with endometrial cancer. Gynecol. Oncol. 2009, 114, 128–134. [Google Scholar] [CrossRef] [Green Version]
- Umar, A.; Boland, C.R.; Terdiman, J.P.; Syngal, S.; de la Chapelle, A.; Rüschoff, J.; Fishel, R.; Lindor, N.M.; Burgart, L.J.; Hamelin, R.; et al. Revised bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J. Natl. Cancer Inst. 2004, 96, 261–268. [Google Scholar] [CrossRef]
- Pai, R.K.; Pai, R.K. A practical approach to the evaluation of gastrointestinal tract carcinomas for Lynch Syndrome. Am. J. Surg. Pathol. 2016, 40, e17–e34. [Google Scholar] [CrossRef] [PubMed]
- de la Chapelle, A.; Palomaki, G.; Hampel, H. Identifying Lynch syndrome. Int. J. Cancer 2009, 125, 1492–1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, K.H.; Ring, K.L. One size may not fit all: The debate of universal tumor testing for Lynch syndrome. Gynecol. Oncol. 2015, 137, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Syngal, S.; Brand, R.E.; Church, J.M.; Giardiello, F.M.; Hampel, H.L.; Burt, R.W. American college of gastroenterology ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am. J. Gastroenterol. 2015, 110, 223–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, J.Y.; Mills, A.M.; Mahadevan, M.S.; Fan, J.; Culp, S.H.; Thomas, M.H.; Cathro, H.P. Universal Lynch Syndrome screening should be performed in all upper tract urothelial carcinomas. Am. J. Surg. Pathol. 2018, 42, 1549–1555. [Google Scholar] [CrossRef]
- Bruegl, A.S.; Djordjevic, B.; Urbauer, D.L.; Westin, S.N.; Soliman, P.T.; Lu, K.H.; Luthra, R.; Broaddus, R.R. Utility of MLH1 methylation analysis in the clinical evaluation of Lynch Syndrome in women with endometrial cancer. Curr. Pharm. Des. 2014, 20, 1655–1663. [Google Scholar] [CrossRef] [Green Version]
- Plon, S.E.; Eccles, D.M.; Easton, D.; Foulkes, W.D.; Genuardi, M.; Greenblatt, M.S.; Hogervorst, F.B.L.; Hoogerbrugge, N.; Spurdle, A.B.; Tavtigian, S.V.; et al. Sequence variant classification and reporting: Recommendations for improving the interpretation of cancer susceptibility genetic test results. Hum. Mutat. 2008, 29, 1282–1291. [Google Scholar] [CrossRef] [Green Version]
- Mas-Moya, J.; Dudley, B.; Brand, R.E.; Thull, D.; Bahary, N.; Nikiforova, M.N.; Pai, R.K. Clinicopathological comparison of colorectal and endometrial carcinomas in patients with Lynch-like syndrome versus patients with Lynch syndrome. Hum. Pathol. 2015, 46, 1616–1625. [Google Scholar] [CrossRef]
- Watkins, J.C.; Yang, E.J.; Muto, M.G.; Feltmate, C.M.; Berkowitz, R.S.; Horowitz, N.S.; Syngal, S.; Yurgelun, M.B.; Chittenden, A.; Hornick, J.L.; et al. Universal screening for mismatch-repair deficiency in endometrial cancers to identify patients with Lynch Syndrome and Lynch-like Syndrome. Int. J. Gynecol. Pathol. 2017, 36, 115–127. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Li, J.; Cragun, J.; Hatch, K.; Chambers, S.K.; Zheng, W. Lynch syndrome related endometrial cancer: Clinical significance beyond the endometrium. J. Hematol. Oncol. J. Hematol. Oncol. 2013, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Nagle, C.M.; O’Mara, T.A.; Tan, Y.; Buchanan, D.D.; Obermair, A.; Blomfield, P.; Quinn, M.A.; Webb, P.M.; Spurdle, A.B. Australian endometrial cancer study group endometrial cancer risk and survival by tumor MMR status. J. Gynecol. Oncol. 2018, 29, e39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niskakoski, A.; Pasanen, A.; Lassus, H.; Renkonen-Sinisalo, L.; Kaur, S.; Mecklin, J.-P.; Bützow, R.; Peltomäki, P. Molecular changes preceding endometrial and ovarian cancer: A study of consecutive endometrial specimens from Lynch syndrome surveillance. Mod. Pathol. 2018, 31, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Dashti, S.G.; Chau, R.; Ouakrim, D.A.; Buchanan, D.D.; Clendenning, M.; Young, J.P.; Winship, I.M.; Arnold, J.; Ahnen, D.J.; Haile, R.W.; et al. Female hormonal factors and the risk of endometrial cancer in Lynch Syndrome. JAMA 2015, 314, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Sato, N.; Sugawara, T.; Kato, A.; Sato, T.; Shimizu, D.; Tamura, D.; Kito, M.; Makino, K.; Shirasawa, H.; et al. Clinical characteristics of Lynch-like cases collaterally classified by Lynch syndrome identification strategy using universal screening in endometrial cancer. Gynecol. Oncol. 2017, 147, 388–395. [Google Scholar] [CrossRef]
- Garg, K.; Leitao, M.M.; Kauff, N.D.; Hansen, J.; Kosarin, K.; Shia, J.; Soslow, R.A. Selection of endometrial carcinomas for dna mismatch repair protein immunohistochemistry using patient age and tumor morphology enhances detection of mismatch repair abnormalities. Am. J. Surg. Pathol. 2009, 33, 925–933. [Google Scholar] [CrossRef]
- Bogani, G.; Tibiletti, M.G.; Ricci, M.T.; Carnevali, I.; Liberale, V.; Paolini, B.; Milione, M.; Vitellaro, M.; Murgia, F.; Chiappa, V.; et al. Lynch syndrome-related non-endometrioid endometrial cancer: Analysis of outcomes. Int. J. Gynecol. Cancer 2020, 30, 56–61. [Google Scholar] [CrossRef]
- Rossi, L.; Le Frere-Belda, M.-A.; Laurent-Puig, P.; Buecher, B.; De Pauw, A.; Stoppa-Lyonnet, D.; Canlorbe, G.; Caron, O.; Borghese, B.; Colas, C.; et al. Clinicopathologic characteristics of endometrial cancer in Lynch Syndrome: A french multicenter study. Int. J. Gynecol. Cancer 2017, 27, 953–960. [Google Scholar] [CrossRef]
- Cohen, R.; Cervera, P.; Svrcek, M.; Pellat, A.; Dreyer, C.; de Gramont, A.; André, T. BRAF-Mutated colorectal cancer: What is the optimal strategy for treatment? Curr. Treat. Options Oncol. 2017, 18, 9. [Google Scholar] [CrossRef]
- Daniels, M.S.; Lu, K.H. Genetic predisposition in gynecologic cancers. Adv. Inherit. Cancers 2016, 43, 543–547. [Google Scholar] [CrossRef]
- Lee, T.S.; Jung, J.Y.; Kim, J.W.; Park, N.-H.; Song, Y.-S.; Kang, S.-B.; Lee, H.-P. Feasibility of ovarian preservation in patients with early stage endometrial carcinoma. Gynecol. Oncol. 2007, 104, 52–57. [Google Scholar] [CrossRef]
- Dogan, A.; Schultheis, B.; Rezniczek, G.A.; Hilal, Z.; Cetin, C.; Hausler, G.; Tempfer, C.B. Synchronous endometrial and ovarian cancer in young women: Case report and review of the literature. Anticancer Res. 2017, 37, 969–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrone, A.; Girolimetti, G.; Procaccini, M.; Marchio, L.; Livi, A.; Borghese, G.; Porcelli, A.; De Iaco, P.; Gasparre, G. Potential for mitochondrial DNA sequencing in the differential diagnosis of gynaecological malignancies. Int. J. Mol. Sci. 2018, 19, 2048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baiocchi, G. ASO Author reflections: Could ovarian preservation be considered for young women with endometrial cancer? Ann. Surg. Oncol. 2020, 27, 2827–2828. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.D.; Buck, A.M.; Shah, M.; Burke, W.M.; Schiff, P.B.; Herzog, T.J. Safety of Ovarian preservation in premenopausal women with endometrial cancer. J. Clin. Oncol. 2009, 27, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Soslow, R.A. Practical issues related to uterine pathology: Staging, frozen section, artifacts, and Lynch syndrome. Mod. Pathol. Off. JUS Can. Acad. Pathol. Inc. 2016, 29 (Suppl. 1), S59–S77. [Google Scholar] [CrossRef] [Green Version]
- Santoro, A.; Piermattei, A.; Inzani, F.; Angelico, G.; Valente, M.; Arciuolo, D.; Spadola, S.; Martini, M.; Fanfani, F.; Fagotti, A.; et al. Frozen section accurately allows pathological characterization of endometrial cancer in patients with a preoperative ambiguous or inconclusive diagnoses: Our experience. BMC Cancer 2019, 19, 1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrone, A.M.; Di Marcoberardino, B.; Rossi, M.; Pozzati, F.; Pellegrini, A.; Procaccini, M.; Santini, D.; De Iaco, P. Laparoscopic versus laparotomic approach to endometrial cancer. Eur. J. Gynaecol. Oncol. 2012, 33, 376–381. [Google Scholar]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: Diagnosis, treatment and follow-up. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2016, 26, 2–30. [Google Scholar] [CrossRef] [Green Version]




| N | Age (Years) | BMI (Kg/m2) | FIGO Stage | Histology | Grade | Revised Bethesda Criteria | Amsterdam Criteria | MMR IHC Pattern | Germline Variant (Pathogenicity Class) [27] |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 34 | 22 | IIIC2 | Dedifferentiated | 3 | Yes | No | Loss of MSH6 | MSH6: c.3155_3156delAG; p.Glu1052ValfsX13 (C5) |
| 2 | 41 | 21 | IIIC1 | Endometrioid | 2 | Yes | Yes | Loss of MSH2/MSH6 | MSH2: c.2581C > T; p.Gln861Ter (C5) |
| 3 | 40 | 18 | II | Endometrioid | 2 | Yes | Yes | Loss of MSH2/MSH6 | MSH2: deletion exon 1 to 7 (C5) |
| 4 | 43 | 23 | IA | Endometrioid | 2 | Yes | Yes | Loss of MSH2/MSH6 | MSH2: deletion exon 2 (C5) |
| 5 | 43 | 21 | IA | Endometrioid | 2 | Yes | No | Loss of MSH2/MSH6 | MSH2: c.484G > A; p.Gly162Arg (C5) |
| 6 | 51 | 20 | IA | Endometrioid | 1 | No | No | Loss of MSH6 | MSH6: c.393_394delAC; p.Gln132ValfsX2 (C5) |
| 7 | 45 | 19 | IA | Endometrioid | 1 | Yes | No | Loss of MLH1 | MLH1: c.1667 + 2T > G (C4) |
| 8 | 45 | 29 | IA | Endometrioid | 2 | Yes | No | Loss of MSH6 | MSH6: c.1700-1701insA; p.Phe569ValfsX8 (C5) |
| 9 | 35 | 18 | II | Endometrioid | 1 | Yes | Yes | Loss of MSH2/MSH6 | MSH2: c.942 + 3° > T (C5) |
| 10 * | 38 | 19 | IA | Endometrioid | 2 | Yes | No | Loss of MSH2/MSH6 | MSH2: deletion exon 15 (C5) |
| 11 * | 41 | 18 | IA | Endometrioid | 1 | Yes | No | Loss of MSH2/MSH6 | MSH2: deletion exon 15 (C5) |
| 12 | 48 | 23 | IA | Endometrioid | 2 | Yes | No | Loss of MSH6 | MSH6: c.1869_1870insC; p.Gly624ArgfsX5 (C5) |
| 13 | 53 | 21 | IA | Endometrioid | 1 | Yes | Yes | Loss of MLH1/PMS2 | MLH1: c.1409 + 2T > G (C4) |
| 14 | 31 | 19 | IA | Endometrioid | 2 | Yes | Yes | Loss of MSH2/MSH6 | MSH2: deletion exon 3 to 6 (C5) |
| 15 | 62 | 29 | IB | Endometrioid | 2 | Yes | No | Loss of MSH6 | MSH6: c.2194C > T p.Arg732Ter (C5) |
| 16 | 60 | 23 | IA | Endometrioid | 2 | No | No | Loss of MSH6 | MSH6: c.1610_1613delAGTA; p.ys537Ilefs * (C5) |
| 17 | 58 | 19 | IA | Endometrioid | 2 | No | No | Loss of MSH6 | MSH6: c.571_573delCTC; p.Leu191del (C5) |
| 18 | 52 | 29 | IA | Endometrioid | 2 | No | No | Loss of MSH6 | MSH6: c.1700_1701insA; p.Phe569Valfs (C5) |
| Characteristics | All Cases | LS | LLC | MMR-Proficient | p Value |
|---|---|---|---|---|---|
| n = 239 | n = 18 | n = 78 | n = 129 | ||
| Bethesda Criteria | <0.001 | ||||
| Yes | 72 (30%) | 14 (78%) | 9 (12%) | 49 (34%) | |
| No | 155 (65%) | 4 (22%) | 64 (82%) | 87 (61%) | |
| NA | 12 (5%) | 0 | 5 (6%) | 7 (5%) | |
| Family Cancer History | 0.002 | ||||
| Yes | 126 (53%) | 16 (89%) | 32 (41%) | 78 (55%) | |
| No | 96 (40%) | 2 (11%) | 41 (53%) | 53 (37%) | |
| NA | 17 (7%) | 0 | 5 (6%) | 12 (8%) | |
| Personal Cancer History | 0.068 | ||||
| Yes | 38 (16%) | 2 (11%) | 7 (9%) | 29 (20%) | |
| No | 199 (83%) | 16 (89%) | 71 (91%) | 112 (79%) | |
| NA | 2 (1%) | 0 | 0 | 2 (1%) | |
| Colorectal Adenomas | <0.001 | ||||
| Yes | 12 (5%) | 5 (28%) | 1 (1%) | 6 (4%) | |
| No | 227 (95%) | 13 (72%) | 77 (99%) | 137 (96%) | |
| Mean Age *, Years (±SD) | 59.2 (±11.3) | 45.5 (±9.0) | 63.2 (±9.6) | 58.8 (±11.1) | <0.001 |
| BMI Kg/m2, Mean(±SD) | 27.9 (±8.4) | 21.8 (±3.3) | 28.3 (±6.3) | 28.4 (±9.5) | 0.006 |
| Mean Age **, Years (±SD) | 51.3 (±4.2) | 48 (±4.0) | 51 (±3.5) | 51.4 (±4.6) | 0.212 |
| HRT | 0.038 | ||||
| Yes | 32 (13%) | 0 | 16 (20%) | 16 (11%) | |
| No | 203 (85%) | 18 (100%) | 62 (80%) | 123 (86%) | |
| NA | 4 (2%) | 0 | 0 | 4 (3%) | |
| COC | <0.001 | ||||
| Yes | 36 (15%) | 9 (50%) | 11 (14%) | 16 (11%) | |
| No | 199 (83%) | 9 (50%) | 67 (86%) | 123 (86%) | |
| NA | 4 (2%) | 0 | 0 | 4 (3%) | |
| Parity | 0.021 | ||||
| Nulliparous | 68 (28%) | 10 (56%) | 18 (23%) | 40 (28%) | |
| Parous | 168 (70%) | 8 (44%) | 60 (77%) | 100 (70%) | |
| NA | 3 (2%) | 0 | 0 | 3 (2%) |
| Characteristics | All Cases | LS | LLC | MMR-Proficient | p Value |
|---|---|---|---|---|---|
| All | n = 239 | n = 18 | n = 78 | n = 129 | |
| Surgical Approach | 0.38 | ||||
| Minimally-invasive | 165 (69%) | 14 (78%) | 50 (64%) | 101 (71%) | |
| Laparotomy | 72 (30%) | 4 (22%) | 36 (36%) | 40 (28%) | |
| Fertility sparing | 2 (1%) | 0 | 0 | 2 (1%) | |
| Hysterectomy | 0.51 | ||||
| Yes | 237 (99%) | 18 (100%) | 78 (100%) | 141 (99%) | |
| No | 2 (1%) | 0 | 0 | 2 (1%) | |
| BSO | 0.07 | ||||
| Yes | 224 (94%) | 17 (94%) | 77 (99%) | 130 (91%) | |
| No | 15 (6%) | 1 (6%) | 1 (1%) | 13 (9%) | |
| Staging Lymphadenectomy | 0.02 | ||||
| Yes | 160 (67%) | 14 (78%) | 60 (77%) | 86 (60%) | |
| No | 79 (33%) | 4 (22%) | 18 (23%) | 57 (40%) | |
| Omentectomy | 0.75 | ||||
| Yes | 42 (18%) | 4 (22%) | 12 (15%) | 26 (18%) | |
| No | 197 (82%) | 14 (78%) | 66 (85%) | 117 (82%) | |
| Histology | 0.312 | ||||
| Endometrioid | 205 (86%) | 17 (94%) | 66 (85%) | 122 (86%) | |
| Dedifferentiated | 20 (8%) | 1 (6%) | 10 (13%) | 9 (6%) | |
| Serous | 12 (5%) | 0 | 2 (2%) | 10 (7%) | |
| Clear Cell | 2 (1%) | 0 | 0 | 2 (1%) | |
| Pattern of Growth | 0.012 | ||||
| Focal | 141 (59%) | 6 (33%) | 54 (69%) | 81 (57%) | |
| Multifocal | 95 (39%) | 12 (67%) | 24 (31%) | 59 (41%) | |
| NA | 3 (2%) | 0 | 0 | 3 (2%) | |
| FIGO Stage | 0.042 | ||||
| IA | 146 (61%) | 13 (72%) | 37 (47%) | 96 (67%) | |
| IB | 44 (18%) | 1 (6%) | 22 (29%) | 21 (15%) | |
| II | 13 (6%) | 2 (11%) | 4 (5%) | 7 (5%) | |
| III/IV | 36 (15%) | 2 (11%) | 15 (19%) | 19 (13%) | |
| Grade | 0.331 | ||||
| Low | 196 (82%) | 17 (94%) | 62 (79%) | 117 (82%) | |
| High | 43 (18%) | 1 (6%) | 16 (21%) | 26 (18%) | |
| LVSI | 0.050 | ||||
| Yes | 71 (30%) | 7 (39%) | 30 (38%) | 34 (24%) | |
| No | 168 (70%) | 11 (61%) | 48 (62%) | 109 (76%) | |
| LN Metastasis | 0.220 | ||||
| Yes | 28 (17%) | 1 (7%) | 14 (23%) | 13 (15%) | |
| No | 134 (83%) | 14 (93%) | 46 (77%) | 74 (85%) | |
| Tubal Lesions | 0.191 | ||||
| Yes | 48 (20%) | 1 (6%) | 19 (24%) | 28 (20%) | |
| No | 191 (80%) | 17 (94%) | 59 (76%) | 115 (80%) | |
| Synchronous EC-OC | 0.360 | ||||
| Yes | 17 (7%) | 2 (11%) | 3 (4%) | 12 (8%) | |
| No | 222 (93%) | 16 (89%) | 75 (96%) | 131 (92%) | |
| Endometriosis | 0.37 | ||||
| Yes | 48 (20%) | 5 (28%) | 12 (15%) | 31 (22%) | |
| No | 191 (80%) | 13 (72%) | 66 (85%) | 112 (78%) | |
| Adjuvant Therapies | 0.01 | ||||
| Yes | 113 (47%) | 9 (50%) | 47 (60%) | 57 (40%) | |
| No | 126 (53%) | 9 (50%) | 31 (40%) | 86 (60%) | |
| Type of Therapy | 0.17 | ||||
| RT | 48 (20%) | 5 (28%) | 20 (26%) | 23 (16%) | |
| CHT | 23 (10%) | 2 (11%) | 5 (6%) | 16 (11%) | |
| RT + CHT | 42 (18%) | 2 (11%) | 22 (28%) | 18 (13%) | |
| Recurrence | 0.51 | ||||
| Yes | 17 (7%) | 2 (11%) | 7 (9%) | 8 (6%) | |
| No | 222 (93%) | 16 (89%) | 71 (91%) | 135 (94%) | |
| Site of Recurrence | 0.53 | ||||
| Local (pelvic) | 11 (65%) | 2 (100%) | 4 (57%) | 5 (63%) | |
| Distant | 6 (35%) | 0 | 3 (43%) | 3 (37%) |
| Characteristics | Total | LS | LLC | MMR-Proficient |
|---|---|---|---|---|
| n = 239 | n = 18 | n = 78 | n = 143 | |
| Histotype Concordance (Bx/His) | ||||
| Yes | 169 (71%) | 13 (72%) | 50 (64%) | 106 (74%) |
| No | 27 (11%) | 1 (6%) | 11 (14%) | 15 (10%) |
| NA | 43 (18%) | 4 (22%) | 17 (22%) | 22 (15%) |
| k Cohen | k = 0.4 | / | k = 0.3 | k = 0.5 |
| p value | p < 0.001 | / | p < 0.001 | p < 0.001 |
| Histotype Concordance (FS/His) | ||||
| Yes | 88 (37%) | 4 (22%) | 19 (24%) | 65 (45%) |
| No | 5 (2%) | 1 (6%) | 2 (3%) | 2 (1%) |
| NA | 146 (61%) | 13 (72%) | 57 (73%) | 76 (53%) |
| k Cohen | k = 0.4 | / | k = 0.6 | / |
| p value | p < 0.001 | / | p < 0.001 | / |
| Grade Concordance (Bx/His) | ||||
| Yes | 122 (51%) | 9 (50%) | 34 (44%) | 79 (55%) |
| No | 43 (18%) | 4 (22%) | 15 (19%) | 24 (17%) |
| NA | 74 (31%) | 5 (28%) | 29 (37%) | 40 (28%) |
| k Cohen | k = 0.5 | k = 0.5 | k = 0.5 | k = 0.6 |
| p value | p < 0.001 | p = 0.03 | p < 0.001 | p < 0.001 |
| Grade Concordance (FS/His) | ||||
| Yes | 74 (31%) | 4 (22%) | 13 (17%) | 57 (40%) |
| No | 11 (5%) | 0 | 4 (5%) | 7 (5%) |
| NA | 154 (64%) | 14 (78%) | 61 (78%) | 79 (55%) |
| k Cohen | k = 0.7 | k = 1 | k = 0.5 | k = 0.8 |
| p value | p < 0.001 | p = 0.04 | p = 0.01 | p < 0.001 |
| Characteristics | Total | MMR-Deficient | MMR-Proficient | p Value |
|---|---|---|---|---|
| n = 239 | n = 96 | n = 143 | ||
| BSO | 0.05 | |||
| Yes | 224 (94%) | 94 (98%) | 130 (91%) | |
| No | 15 (6%) | 2 (2%) | 13 (9%) | |
| Lymph Nodes Analysis | 0.01 | |||
| Positive | 160 (67%) | 74 (77%) | 86 (60%) | |
| Negative | 79 (33%) | 22 (23%) | 57 (40%) | |
| LVSI | 0.01 | |||
| Yes | 71 (30%) | 37 (39%) | 34 (24%) | |
| No | 168 (70%) | 59 (61%) | 109 (76%) | |
| Adjuvant Therapies | 0.005 | |||
| Yes | 113 (47%) | 56 (58%) | 57 (40%) | |
| No | 126 (53%) | 40 (42%) | 86 (60%) | |
| COC | 0.038 | |||
| Yes | 36 (15%) | 20 (21%) | 16 (11%) | |
| No | 199 (83%) | 76 (79%) | 123 (86%) | |
| NA | 4 (2%) | 0 (0%) | 4 (3%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dondi, G.; Coluccelli, S.; De Leo, A.; Ferrari, S.; Gruppioni, E.; Bovicelli, A.; Godino, L.; Coadă, C.A.; Morganti, A.G.; Giordano, A.; et al. An Analysis of Clinical, Surgical, Pathological and Molecular Characteristics of Endometrial Cancer According to Mismatch Repair Status. A Multidisciplinary Approach. Int. J. Mol. Sci. 2020, 21, 7188. https://doi.org/10.3390/ijms21197188
Dondi G, Coluccelli S, De Leo A, Ferrari S, Gruppioni E, Bovicelli A, Godino L, Coadă CA, Morganti AG, Giordano A, et al. An Analysis of Clinical, Surgical, Pathological and Molecular Characteristics of Endometrial Cancer According to Mismatch Repair Status. A Multidisciplinary Approach. International Journal of Molecular Sciences. 2020; 21(19):7188. https://doi.org/10.3390/ijms21197188
Chicago/Turabian StyleDondi, Giulia, Sara Coluccelli, Antonio De Leo, Simona Ferrari, Elisa Gruppioni, Alessandro Bovicelli, Lea Godino, Camelia Alexandra Coadă, Alessio Giuseppe Morganti, Antonio Giordano, and et al. 2020. "An Analysis of Clinical, Surgical, Pathological and Molecular Characteristics of Endometrial Cancer According to Mismatch Repair Status. A Multidisciplinary Approach" International Journal of Molecular Sciences 21, no. 19: 7188. https://doi.org/10.3390/ijms21197188