Minor Ginsenoside Rg2 and Rh1 Attenuates LPS-Induced Acute Liver and Kidney Damages via Downregulating Activation of TLR4-STAT1 and Inflammatory Cytokine Production in Macrophages
Abstract
:1. Introduction
2. Results
2.1. Effect of the Ginsenosides Rg2 and Rh1 on Cell Viability and Inflammatory Mediator Production
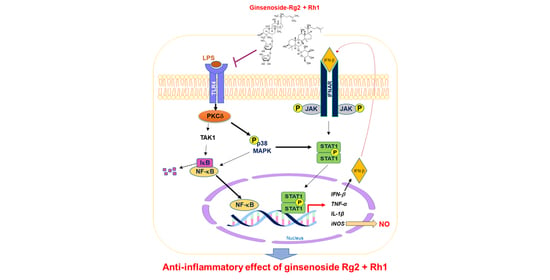
2.2. Inhibitory Effect of the Ginsenosides Rg2 and Rh1 on LPS-TLR4 Binding on Macrophages
2.3. Inhibitory Effect of the Ginsenosides Rg2 and Rh1 on the TLR4-Mediated Signaling Pathways in LPS-Stimulated RAW264.7 Cells
2.4. Effect of the Ginsenosides Rg2 and Rh1 on NF-κB p65 Nuclear Translocation and Activation and Cytokine Production
2.5. Effect of the Ginsenosides Rg2 and Rh1 on Pathological Changes and Liver Function in LPS-Challenged Mice
2.6. Effect of the Ginsenosides Rg2 and Rh1 on Pathological Changes and Kidney Function in LPS-Challenged Mice
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Confocal Microscopy
4.5. NO Measurement
4.6. Western Blotting
4.7. Immunofluorescence Staining
4.8. Preparation of Cytosolic Extracts and Nuclear Extracts
4.9. Real-Time Reverse Transcription (RT)-PCR Reaction
4.10. Animal Experiments
4.11. Isolation of Peritoneal Macrophages
4.12. Histological Analysis
4.13. Immunohistochemistry Staining
4.14. Biochemical Analysis
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALT | Alanine transaminase |
| AST | Aspartate transaminase |
| BUN | Blood urea nitrogen |
| CRE | Creatinine |
| ERK 1/2 | Extracellular signal-regulated kinase 1/2 |
| FBS | Fetal bovine serum |
| H&E | Hematoxylin and eosin |
| IL-1β | Interleukin-1 beta |
| iNOS | Inducible nitric oxide synthase |
| JAK | Janus kinase |
| JNK | c-Jun N-terminal kinase |
| LPS | Lipopolysaccharide |
| MTT | 3-(4,5-dimethelthiazon-2-yl)-2,5-diphehnyltetrazolium bromide |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO | Nitric oxide |
| PBS | Phosphate buffered saline |
| PPT | Protopanaxatriol |
| PPD | Protopanaxadiol |
| STAT1 | Signal transducers and activator of transcription |
| TLR4 | Toll-like receptor 4 |
| TNF-α | Tumor necrosis factor-alpha |
References
- Chancharoenthana, W.; Leelahavanichkul, A. Acute Kidney Injury Spectrum in Patients with Chronic Liver Disease: Where Do We Stand? World J. Gastroenterol. 2019, 25, 3684–3703. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, M.; Evans, L.; Levy, M.; Rhodes, A. Sepsis and Septic Shock. Lancet 2018, 392, 75–87. [Google Scholar] [CrossRef]
- Woznica, E.A.; Inglot, M.; Woznica, R.K.; Lysenko, L. Liver Dysfunction in Sepsis. Adv. Clin. Exp. Med. 2018, 27, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, B.; Finfer, S.; Cohen, J.; Rajbhandari, D.; Arabi, Y.; Bellomo, R.; Billot, L.; Correa, M.; Glass, P.; Harward, M.; et al. Adjunctive Glucocorticoid Therapy in Patients with Septic Shock. N. Engl. J. Med. 2018, 378, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 Signal Transduction Pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Rathinam, V.A.K.; Zhao, Y.; Shao, F. Innate Immunity to Intracellular LPS. Nat. Immunol. 2019, 20, 527–533. [Google Scholar] [CrossRef]
- Erridge, C. Endogenous Ligands of TLR2 and TLR4: Agonists or Sssistants? J. Leukoc. Biol. 2010, 87, 989–999. [Google Scholar] [CrossRef]
- He, W.; Qu, T.; Yu, Q.; Wang, Z.; Lv, H.; Zhang, J.; Zhao, X.; Wang, P. LPS Induces IL-8 Expression Through TLR4, MyD88, NF-kappaB and MAPK Pathways in Human Dental Pulp Stem Cells. Int. Endod. J. 2013, 46, 128–136. [Google Scholar] [CrossRef]
- Byun, E.B.; Sung, N.Y.; Byun, E.H.; Song, D.S.; Kim, J.K.; Park, J.H.; Song, B.S.; Park, S.H.; Lee, J.W.; Byun, M.W.; et al. The Procyanidin Trimer C1 Inhibits LPS-induced MAPK and NF-kappaB Signaling through TLR4 in Macrophages. Int. Immunopharmacol. 2013, 15, 450–456. [Google Scholar] [CrossRef]
- Lai, J.L.; Liu, Y.H.; Liu, C.; Qi, M.P.; Liu, R.N.; Zhu, X.F.; Zhou, Q.G.; Chen, Y.Y.; Guo, A.Z.; Hu, C.M. Indirubin Inhibits LPS-Induced Inflammation via TLR4 Abrogation Mediated by the NF-kB and MAPK Signaling Pathways. Inflammation 2017, 40, 1–12. [Google Scholar] [CrossRef]
- Dai, B.; Wei, D.; Zheng, N.N.; Chi, Z.H.; Xin, N.; Ma, T.X.; Zheng, L.Y.; Sumi, R.; Sun, L. Coccomyxa Gloeobotrydiformis Polysaccharide Inhibits Lipopolysaccharide-Induced Inflammation in RAW264.7 Macrophages. Cell. Physiol. Biochem. 2018, 51, 2523–2535. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Yi, Y.S.; Kim, M.Y.; Cho, J.Y. Role of Ginsenosides, the Main Active Components of Panax Ginseng, in Inflammatory Responses and Diseases. J. Ginseng Res. 2017, 41, 435–443. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Ye, Y.; Xiao, L.; Duan, X.; Zhang, Y.; Zhang, H. Anticancer Effects of Ginsenoside Rg3 (Review). Int. J. Mol. Med. 2017, 39, 507–518. [Google Scholar] [CrossRef] [Green Version]
- Baatar, D.; Siddiqi, M.Z.; Im, W.T.; Ul Khaliq, N.; Hwang, S.G. Anti-Inflammatory Effect of Ginsenoside Rh2-Mix on Lipopolysaccharide-Stimulated RAW264.7 Murine Macrophage Cells. J. Med. Food 2018, 21, 951–960. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Chen, K. Roles and Mechanisms of Ginsenoside in Cardiovascular Diseases: Progress and Perspectives. Sci. China Life Sci. 2016, 59, 292–298. [Google Scholar] [CrossRef]
- Li, J.; Zeng, B.; Hu, X.; Li, Z.; Zhang, D.; Yang, G.; Dai, J.; Zeng, X. Protective Effects of Ginsenoside Rb1 against Blood-Brain Barrier Damage Induced by Human Immunodeficiency Virus-1 Tat Protein and Methamphetamine in Sprague-Dawley Rats. Am. J. Chin. Med. 2018, 46, 551–566. [Google Scholar] [CrossRef]
- Tao, T.; Chen, F.; Bo, L.; Xie, Q.; Yi, W.; Zou, Y.; Hu, B.; Li, J.; Deng, X. Ginsenoside Rg1 Protects Mouse Liver Against Ischemia-reperfusion Injury through Anti-inflammatory and Anti-apoptosis Properties. J. Surg. Res. 2014, 191, 231–238. [Google Scholar] [CrossRef]
- Sun, Q.; Meng, Q.T.; Jiang, Y.; Xia, Z.Y. Ginsenoside Rb1 Attenuates Intestinal Ischemia Reperfusion Induced Renal Injury by Activating Nrf2/ARE Pathway. Molecules 2012, 17, 7195–7205. [Google Scholar] [CrossRef]
- Qu, L.; Zhu, Y.; Liu, Y.; Yang, H.; Zhu, C.; Ma, P.; Deng, J.; Fan, D. Protective Effects of Ginsenoside Rk3 Against Chronic Alcohol-induced Liver Injury in Mice Through Inhibition of Inflammation, Oxidative Stress, and Apoptosis. Food Chem. Toxicol. 2019, 126, 277–284. [Google Scholar] [CrossRef]
- Kang, K.S.; Kim, H.Y.; Yamabe, N.; Park, J.H.; Yokozawa, T. Preventive Effect of 20(S)-Ginsenoside Rg3 Against Lipopolysaccharide-Induced Hepatic and Renal Injury in Rats. Free Radic. Res. 2007, 41, 1181–1188. [Google Scholar] [CrossRef]
- Li, W.; Wang, J.Q.; Zhou, Y.D.; Hou, J.G.; Liu, Y.; Wang, Y.P.; Gong, X.J.; Lin, X.H.; Jiang, S.; Wang, Z. Rare Ginsenoside 20(R)-Rg3 Inhibits D-Galactose-Induced Liver and Kidney Injury by Regulating Oxidative Stress-Induced Apoptosis. Am. J. Chin. Med. 2020, 48, 1141–1157. [Google Scholar] [CrossRef]
- Liu, X.; Mi, X.; Wang, Z.; Zhang, M.; Hou, J.; Jiang, S.; Wang, Y.; Chen, C.; Li, W. Ginsenoside Rg3 Promotes Regression from Hepatic Fibrosis Through Reducing Inflammation-Mediated Autophagy Signaling Pathway. Cell Death Dis. 2020, 11, 454. [Google Scholar] [CrossRef]
- Kim, D.H. Gut Microbiota-Mediated Pharmacokinetics of Ginseng Saponins. J. Ginseng Res. 2018, 42, 255–263. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, J.L.; Zhang, X.; Wang, H.; Ye, Y.; Song, L.; Wang, Y.J.; Tu, M.J.; Wang, W.W.; Yang, L.; et al. Antidepressant-Like Effects of Ginsenoside Rg2 in a Chronic Mild Stress Model of Depression. Brain Res. Bull. 2017, 134, 211–219. [Google Scholar] [CrossRef]
- Liu, H.; Liu, M.; Jin, Z.; Yaqoob, S.; Zheng, M.; Cai, D.; Liu, J.; Guo, S. Ginsenoside Rg2 Inhibits Adipogenesis in 3T3-L1 Preadipocytes and Suppresses Obesity in High-Fat-Diet-Induced Obese Mice Through the AMPK Pathway. Food Funct. 2019, 10, 3603–3614. [Google Scholar] [CrossRef]
- Jung, J.S.; Shin, J.A.; Park, E.M.; Lee, J.E.; Kang, Y.S.; Min, S.W.; Kim, D.H.; Hyun, J.W.; Shin, C.Y.; Kim, H.S. Anti-Inflammatory Mechanism of Ginsenoside Rh1 in Lipopolysaccharide-Stimulated Microglia: Critical Role of the Protein Kinase A Pathway and Hemeoxygenase-1 Expression. J. Neurochem. 2010, 115, 1668–1680. [Google Scholar] [CrossRef]
- Jung, J.S.; Ahn, J.H.; Le, T.K.; Kim, D.H.; Kim, H.S. Protopanaxatriol Ginsenoside Rh1 Inhibits the Expression of Matrix Metalloproteinases and the in Vitro Invasion/Migration of Human Astroglioma Cells. Neurochem. Int. 2013, 63, 80–86. [Google Scholar] [CrossRef]
- Jeong, J.J.; Kim, B.; Kim, D.H. Ginsenoside Rh1 Eliminates the Cytoprotective Phenotype of Human Immunodeficiency Virus Type 1-transduced hHuman Macrophages by Inhibiting the Phosphorylation of Pyruvate Dehydrogenase Lipoamide Kinase Isozyme 1. Biol. Pharm. Bull. 2013, 36, 1088–1094. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.D.; Kim, D.Y.; Quan, H.Y.; Kim, S.J.; Jung, M.S.; Chung, S.H. Ginsenoside Rg2 Induces Orphan Nuclear Receptor SHP Gene Expression and Inactivates GSK3beta via AMP-Activated Protein Kinase to Inhibit Hepatic Glucose Production in HepG2 Cells. Chem. Biol. Interact. 2012, 195, 35–42. [Google Scholar] [CrossRef]
- Jung, J.S.; Kim, D.H.; Kim, H.S. Ginsenoside Rh1 Suppresses Inducible Nitric Oxide Synthase Gene Expression in IFN-Gamma-Stimulated Microglia via Modulation of JAK/STAT and ERK Signaling Pathways. Biochem. Biophys. Res. Commun. 2010, 397, 323–328. [Google Scholar] [CrossRef]
- Quan, L.H.; Min, J.W.; Sathiyamoorthy, S.; Yang, D.U.; Kim, Y.J.; Yang, D.C. Biotransformation of Ginsenosides Re and Rg1 into Ginsenosides Rg2 and Rh1 by Recombinant Beta-Glucosidase. Biotechnol. Lett. 2012, 34, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Kim, C.H.; Ha, T.S.; Lee, S.J.; Ahn, H.Y. Ginsenoside Rg2 Inhibits Lipopolysaccharide-Induced Adhesion Molecule Expression in Human Umbilical Vein Endothelial Cell. Korean J. Physiol. Pharm. 2013, 17, 133–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najjar, I.; Fagard, R. STAT1 and Pathogens, not a Friendly Relationship. Biochimie 2010, 92, 425–444. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Jeong, J.J.; Eun, S.H.; Kim, D.H. Anti-Inflammatory Effects of Ginsenoside Rg1 and its Metabolites Ginsenoside Rh1 and 20(S)-Protopanaxatriol in Mice with TNBS-Induced Colitis. Eur. J. Pharm. 2015, 762, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Luu, K.; Greenhill, C.J.; Majoros, A.; Decker, T.; Jenkins, B.J.; Mansell, A. STAT1 Plays A Role in TLR Signal Transduction and Inflammatory Responses. Immunol. Cell. Biol. 2014, 92, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Ramsauer, K.; Sadzak, I.; Porras, A.; Pilz, A.; Nebreda, A.R.; Decker, T.; Kovarik, P. P38 MAPK Enhances STAT1-Dependent Transcription Independently of Ser-727 Phosphorylation. Proc. Natl. Acad. Sci. USA 2002, 99, 12859–12864. [Google Scholar] [CrossRef] [Green Version]
- Alamuru, N.P.; Behera, S.; Butchar, J.P.; Tridandapani, S.; Kaimal Suraj, S.; Babu, P.P.; Hasnain, S.E.; Ehtesham, N.Z.; Parsa, K.V. A Novel Immunomodulatory Function of PHLPP1: Inhibition of iNOS via Attenuation of STAT1 ser727 Phosphorylation in Mouse Macrophages. J. Leukoc. Biol. 2014, 95, 775–783. [Google Scholar] [CrossRef]
- Fujiwara, N.; Kobayashi, K. Macrophages in Inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef]
- Chaudhry, H.; Zhou, J.; Zhong, Y.; Ali, M.M.; McGuire, F.; Nagarkatti, P.S.; Nagarkatti, M. Role of Cytokines as A Double-Edged Sword in Sepsis. In Vivo 2013, 27, 669–684. [Google Scholar]
- Song, J.D.; Lee, S.K.; Kim, K.M.; Kim, J.W.; Kim, J.M.; Yoo, Y.H.; Park, Y.C. Redox Factor-1 Mediates NF-KappaB Nuclear Translocation for LPS-Induced iNOS Expression in Murine Macrophage Cell Line RAW 264.7. Immunology 2008, 124, 58–67. [Google Scholar] [CrossRef]
- Jin, Y.; Huynh, D.T.N.; Kang, K.W.; Myung, C.S.; Heo, K.S. Inhibition of p90RSK Activation Sensitizes Triple-Negative Breast Cancer Cells to Cisplatin by Inhibiting Proliferation, Migration and EMT. BMB Rep. 2019, 52, 706–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huynh, D.T.N.; Baek, N.; Sim, S.; Myung, C.-S.; Heo, K.-S. Minor Ginsenoside Rg2 and Rh1 Attenuates LPS-Induced Acute Liver and Kidney Damages via Downregulating Activation of TLR4-STAT1 and Inflammatory Cytokine Production in Macrophages. Int. J. Mol. Sci. 2020, 21, 6656. https://doi.org/10.3390/ijms21186656
Huynh DTN, Baek N, Sim S, Myung C-S, Heo K-S. Minor Ginsenoside Rg2 and Rh1 Attenuates LPS-Induced Acute Liver and Kidney Damages via Downregulating Activation of TLR4-STAT1 and Inflammatory Cytokine Production in Macrophages. International Journal of Molecular Sciences. 2020; 21(18):6656. https://doi.org/10.3390/ijms21186656
Chicago/Turabian StyleHuynh, Diem Thi Ngoc, Naehwan Baek, Sohyun Sim, Chang-Seon Myung, and Kyung-Sun Heo. 2020. "Minor Ginsenoside Rg2 and Rh1 Attenuates LPS-Induced Acute Liver and Kidney Damages via Downregulating Activation of TLR4-STAT1 and Inflammatory Cytokine Production in Macrophages" International Journal of Molecular Sciences 21, no. 18: 6656. https://doi.org/10.3390/ijms21186656