The Correlation between the Water Content and Electrolyte Permeability of Cation-Exchange Membranes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Equilibrium Properties of the Membranes
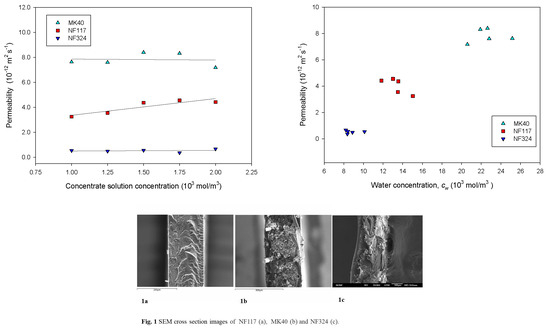
2.2. Transport Properties of the Membranes
3. Experimental Section
3.1. Membranes
3.2. Permeation Measurements
3.3. Water Content
4. Basic Equations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of ion Exchange membranes: A Review. J. Memb. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Xu, Z.; Yang, Z. Poly(phenylene oxide)-Based Ion-Exchange Membranes for Aqueous Organic Redox Flow Battery. Ind. Eng. Chem. Res. 2019, 58, 10707–10712. [Google Scholar] [CrossRef]
- Rottiers, T.; Van der Bruggen, B.; Pinoy, L. Permeability of Small Alcohols through Commercial Ion-Exchange Membranes Used in Electrodialysis. Ind. Eng. Chem. Res. 2016, 55, 8215–8224. [Google Scholar] [CrossRef]
- Li, J.; Zhu, J.; Yuan, S.; Lin, J.; Shen, J.; Van der Bruggen, B. Cation-Exchange Membranes with Controlled Porosity in Electrodialysis Application. Ind. Eng. Chem. Res. 2017, 56, 8111–8120. [Google Scholar] [CrossRef]
- Tanaka, Y. Ion-Exchange Membrane Electrodialysis for Saline Water Desalination and Its Application to Seawater Concentration. Ind. Eng. Chem. Res. 2011, 50, 7494–7503. [Google Scholar] [CrossRef]
- Strathmann, H.; Grabowski, A.; Eigenberger, G. Ion-Exchange Membranes in the Chemical Process Industry. Ind. Eng. Chem. Res. 2013, 52, 10364–10379. [Google Scholar] [CrossRef]
- Koter, S.; Piotrowski, P.; Kerres, J. Comparative investigations of ion Exchange membranes. J. Memb. Sci. 1999, 153, 83–90. [Google Scholar] [CrossRef]
- Ran, J.; Wu, L.; He, Y.; Yang, X.; Xu, T. Ion Exchange membranes: New developements and applications. J. Memb. Sci. 2017, 522, 267–291. [Google Scholar] [CrossRef]
- Okada, T.; Xie, G.; Gorseth, O.; Kjelstrup, S.; Arimura, T. Ion and water transport characteristics of Nafion membranes as electrolytes. Electrochim. Acta. 1998, 43, 3741–3747. [Google Scholar] [CrossRef]
- Stenina, I.A.; Sistat, P.; Rebrov, A.I.; Pourcelly, G.; Yaroslavtsev, A.B. Ion mobility in Nafion-117 membranes. Desalination 2004, 170, 49–57. [Google Scholar] [CrossRef]
- Koter, S.; Zator, M. Determination of the electrolyte and osmotic permeability coefficients by conductometric and emf methods. Desalination 2004, 162, 373–381. [Google Scholar] [CrossRef]
- Cañas, A.; Ariza, M.J.; Benavente, J. A Comparison of Electrochemical and Electrokinetic Parameters Determined for Cellophane Membranes in Contact with NaCl and NaNO3 Solutions. J. Colloid Interface Sci. 2002, 246, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Berezina, N.P.; Kononenko, N.A.; Dyomina, O.A.; Gnusin, N.P. Characterization of ion-exchange membrane materials: Properties vs. structure. Adv. Colloid Interf. Sci. 2008, 139, 3–28. [Google Scholar] [CrossRef] [PubMed]
- Filippov, A.N.; Starov, V.M.; Kononenko, N.A.; Berezina, N.P. Asymmetry of diffusion permeability of bi-layer membranes. Adv. Colloid Interf. Sci. 2008, 139, 29–44. [Google Scholar] [CrossRef]
- Barragan, V.M.; Villaluenga, J.P.G.; Godino, M.P.; Izquierdo-Gil, M.A.; Ruiz-Bauzá, C.; Seoane, B. Experimental estimation of equilibrium and transport properties of sulfonated cation-exchange membranes with different morphologies. J. Colloid Interface Sci. 2009, 333, 497–502. [Google Scholar] [CrossRef]
- Nandan, D.; Mohan, H.; Iyer, R.M. Methanol and water uptake, densities, equivalental volumes and thicknesses of several uni- and divalent ionic perfluorosulphonate exchange membranes (Nafion-117) and their methanol-water fractionation behaviour at 298 K. J. Memb. Sci. 1992, 71, 69–80. [Google Scholar] [CrossRef]
- Larchet, C.; Auclair, B.; Nikonenko, V. Approximate evaluation of water transport number in ion-exchange membranes. Electrochim. Acta. 2004, 49, 1711–1717. [Google Scholar] [CrossRef]
- Volodina, E.; Pismenskaya, N.; Nikonenko, V.; Larchet, C.; Pourcelly, G. Ion transfer cross ion-exchange membranes with homogeneous and heterogeneous surfaces. J. Colloid Interface Sci. 2005, 285, 247–258. [Google Scholar] [CrossRef]
- Lehmani, A.; Turq, P.; Périé, M.; Périé, J.; Simonin, J.-P. Ion transport in Nation ® 117 membrane. J. Electroanal. Chem. 1997, 428, 81–89. [Google Scholar] [CrossRef]
- Helfferich, F. Ion Exchange; McGraw-Hill: New York, NY, USA, 1962. [Google Scholar]
- Kozmai, A.E.; Nikonenko, V.V.; Zyryanova, S.; Pismenskaya, N.D.; Dammak, L. A simple model for the response of an anion-exchange membrane to variation in concentration and pH of bathing solution. J. Memb. Sci. 2018, 567, 127–138. [Google Scholar] [CrossRef]
- Filippov, A.N.; Kononenko, N.A.; Demina, O.A. Diffusion of Electrolytes of different natures through the Cation-Exchange Membrane. Colloid J. 2017, 79, 556–566. [Google Scholar] [CrossRef]
- Demina, O.A.; Kononenko, N.A.; Falina, I.A.; Demin, A.V. Theoretical Estimation of Differential Coefficients of Ion-Exchange Membrane Diffusion Permeability. Colloid J. 2017, 79, 317–327. [Google Scholar] [CrossRef]
- Sarapulova, V.V.; Titorova, V.D.; Nikonenko, V.V.; Pismenskaya, N.D. Transport Characteristics of Homogeneous and Heterogeneous Ion-Exchange Membranes in Sodium Chloride, Calcium Chloride and Sodium Sulfate Solutions. Membr. Membr. Technol. 2019, 1, 198–213. [Google Scholar] [CrossRef]
- Kingsbury, R.S.; Zhu, S.; Flotron, S.; Coronell, O. Microstructure Determines Water and Salt Permeation in Commercial Ion-Exchange Membranes. ACS Appl. Mater. Interfaces 2018, 10, 39745–39756. [Google Scholar] [CrossRef] [PubMed]
- Kingsbury, R.S.; Bruning, K.; Zhu, S.; Flotron, S.; Miller, C.T.; Coronell, O. Influence of water uptake, charge, manning parameter, and contact angle on water and salt transport in commercial ion exchange membranes. Ind. Eng. Res. 2019, 58, 18663–18674. [Google Scholar] [CrossRef]
- Izquierdo-Gil, M.A.; Barragán, V.M.; Villaluenga, J.P.G.; Godino, M.P. Water uptake and salt transport through Nafion cation-exchange membranes with different thicknesses. Chem. Eng. Sci. 2011, 72, 1–9. [Google Scholar] [CrossRef]






| NF117 | MK-40 | NF324 | |
|---|---|---|---|
| Thickness a (10−6 m) | 186 | 450 | 271 |
| Density a (103 kg/m3) | 1.98 | 1.12 | 1.55 |
| IEC b (meq/g) | 1.1 | 1.7 | 0.92 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izquierdo-Gil, M.A.; Villaluenga, J.P.G.; Muñoz, S.; Barragán, V.M. The Correlation between the Water Content and Electrolyte Permeability of Cation-Exchange Membranes. Int. J. Mol. Sci. 2020, 21, 5897. https://doi.org/10.3390/ijms21165897
Izquierdo-Gil MA, Villaluenga JPG, Muñoz S, Barragán VM. The Correlation between the Water Content and Electrolyte Permeability of Cation-Exchange Membranes. International Journal of Molecular Sciences. 2020; 21(16):5897. https://doi.org/10.3390/ijms21165897
Chicago/Turabian StyleIzquierdo-Gil, M. A., J. P. G. Villaluenga, S. Muñoz, and V. M. Barragán. 2020. "The Correlation between the Water Content and Electrolyte Permeability of Cation-Exchange Membranes" International Journal of Molecular Sciences 21, no. 16: 5897. https://doi.org/10.3390/ijms21165897