Recent Advances in Porphyrin-Based Materials for Metal Ions Detection
Abstract
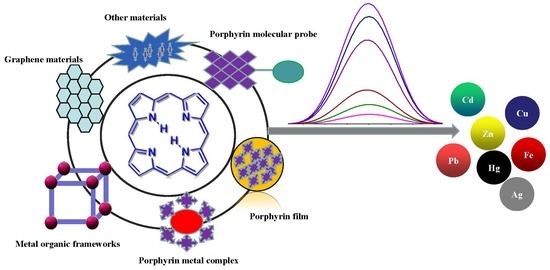
:1. Introduction
2. Porphyrin-Based Fluorescent Molecular Probes for Metal Ions Detection
2.1. Porphyrin Molecule by Functional Modification
2.2. Detecting Mechanism of Fluorescent Porphyrin Molecular Probes
3. Construction of Porphyrin-Based Materials for Metal Ions Detection
3.1. Porphyrin Film
3.2. Porphyrin Metal Complex
3.3. Metal–Organic Frameworks
3.4. Graphene Materials
3.5. Other Materials
4. Conclusions and Perspectives
Funding
Conflicts of Interest
Abbreviations
| 8-HQ-B | 8-Hydroxy Quinoline Benzoate |
| AIE | Aggregation-Induced Luminescence |
| ATRP | Atom Transfer Radical Polymerization |
| Au | Gold |
| Bis-TMPipEOPP | Bis-porphyrin (4,4’-bis[tris[4-[2-(1-methyl-1piperidinyl)ethoxy]phenyl] porphyrin |
| BODIPY | Boron–Dipyrromethene |
| CCG | Chemically Converted Graphene |
| CNCs | Cellulose Nanocrystals |
| CNC-SA-COOC6TPP | Synthesized by facile esterification of extended porphyrin with carboxylated cellulose nanocrystals |
| CP | Coordination Polymer |
| CMPSF | Chloromethylated Polysulfone |
| DMF | Dimethylformamide |
| DPA | 2,2’-dipyridylamine |
| DTPP | 5-p-[[4-(10’,-15’,20’-triphenyl-5’-porphinato)phenyloxyl]-1-butyloxyl]-phenyl-10,15,20-triphenylporphine |
| EDTA | Ethylenediaminetetraacetic acid |
| ESIPT | Excited Intra-molecular proton transfer |
| FRET | Fluorescence Resonance Energy Transfer |
| GO | Graphene Oxide |
| HPLC | High-Performance Liquid Chromatography |
| H2TEHPPS | 5,10,15,20-tetra (3-ethoxy-4-hydroxy-5-sulfonate) phenylporphyrin |
| H2TPPBPy | A porphyrin derivative appended with bipyridine |
| IC | Ion Chromatography |
| ICP-MS | Inductively Coupled Plasma-Mass Spectroscopy |
| ICT | Intra-molecular Charge Transfer |
| IFE | Inner Filter Effect |
| LOD | Limit of Detection |
| MB | Methylene Blue |
| MOF-525 | Porphyrinic MOF Zr6O4(OH)4(TCPP-H2)3 |
| MOFs | Metal–Organic Frameworks |
| MoS2 | Molybdenum Disulfide |
| NGQDs | Nitrogen-doped Graphene Quantum Dots |
| NPs | Nanoparticles |
| PAH | Poly(allylamine hydrochloride) |
| PAN | Polyacrylonitrile |
| Pc | Phthalocyanine |
| PCN-221 | The microporous zirconium-based MOF |
| PCN-222 | Porous coordination network is constructed using 5,10,15,20-tetrakis (4-carboxyphenyl) porphyrin (H2TCPP) as a heme-like ligand and highly stable Zr6 clusters as nodes |
| PCN-224 | The coordination of Zr(IV) metal ions with meso-tetra(4-carboxyphenyl) porphyrin (TCPP) ligands produce MOF PCN-224); PET (Photo-induced Electron Transfer |
| PNaSS/PSS | Poly (sodium 4-styrenesulfonate) |
| Por | Porphyrin |
| PSF | Polysulfone |
| PSF-PNaSS | PNaSS-grafted polysulfone |
| PVC | Polyvinyl Chloride |
| QDs | Quantum Dots |
| SA | Succinic Anhydride |
| SBA-16 | A mesoporous silica synthesized using tetraethoxysilane as a silicon source and a ternary combination of surfactant, water, and butanol |
| TCPP | 5,10,15,20-tetrakis (4-carboxyphenyl) porphyrin |
| TDMPzP | Meso-tetrakis (1,2-dimethylpyrazolium-4-yl) porphyrin sulfonate |
| THPP | 5,10,15,20-tetrakis (4-hydroxyphenyl) porphyrin |
| THMPP | 5,10,15,20-tetrakis (4-hydroxy-3,5-dimethoxyphenyl) porphyrin |
| TMPP | 5,10,15,20-tetrakis (3,4,5-trimethoxyphenyl) porphyrin |
| TMPyP | 5,10,15,20-tetrakis (4-N-methylpyridinyl) porphyrin |
| TPP | Tetraphenylporphyrin |
| TPPS | 5,10,15,20-tetrakis (p-sulfonatophenyl) porphyrin |
| TPP−PZS | Highly cross-linked poly(tetraphenylporphyrin-co-cyclotriphosphazene) |
| TPyP | Tetrakis (4-pyridyl) porphyrin |
| TPyP5-MGs | Tetrakis (4-pyridyl) porphyrin (TPyP) functionalized thermosensitive ion microgel |
| UCNPs | Upconversion Nanoparticles |
| UiO-66(OH)2 | A MOF named by University of Oslo |
| UV | Ultra-Violet |
| ZnP-CONH-Q | Zinc Porphyrin-Quinone-Linked Dyad |
| Zr-MOFs-SH(O) | Mercapto-functionalized Zr-MOFs |
References
- Kim, M. Determination of lead and cadmium in wines by graphite furnace atomic absorption spectrometry. Food Addit. Contam. 2004, 21, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Dong, R.; Yu, K.; Luoab, X.; Tu, X.; Luo, S.; Yang, L. Determination of trace total inorganic arsenic by hydride generation atomic fluorescence spectrometry after solid phase extraction-preconcentration on aluminium hydroxide gel. Microchim. Acta 2013, 180, 509–515. [Google Scholar] [CrossRef]
- Jones, D.R.; Jarrett, J.M.; Tevis, D.S.; Franklin, M.; Mullinix, N.J.; Wallon, K.L.; Derrick Quarles, C., Jr.; Caldwell, K.L.; Jones, R.L. Analysis of whole human blood for Pb, Cd, Hg, Se, and Mn by ICP–DRC–MS for biomonitoring and acute exposures. Talanta 2017, 162, 114–122. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Pang, J.; Wu, Y.; Huang, X. Development of magnetism-reinforced in-tube solid phase microextraction combined with HPLC for the sensitive quantification of cobalt(II) and nickel(II) in environmental waters. Microchem. J. 2020, 105370. [Google Scholar] [CrossRef]
- Safni, S.; Takeuchi, T.; Miwa, T. Application of microcolumn ion chromatography using anion exchangers modified with dextran sulfate for the determination of alkali and alkaline-earth metal ions. J. Chromatogr. B Biomed. Sci. Appl. 2001, 753, 409–412. [Google Scholar] [CrossRef]
- Li, Y.; Hou, J.; Zhou, H.-P.; Jia, M.; Chen, S.; Huang, H.; Zhang, L.; Yu, C. A fluorescence sensor array based on perylene probe monomer-excimer emission transition for the highly efficient differential sensing of metal ions and drinking waters. Sens. Actuators B Chem. 2020, 319, 128212. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Sivanesan, S.; Panneerselvam, P. Turn-on fluorescence sensor based detection of heavy metal ion using carbon dots@graphitic-carbon nitride nanocomposite probe. J. Photochem. Photobiol. A Chem. 2020, 389, 112204. [Google Scholar] [CrossRef]
- Rana, M.; Chowdhury, P. L-glutathione capped CdSeS/ZnS quantum dot sensor for the detection of environmentally hazardous metal ions. J. Lumin. 2019, 206, 105–112. [Google Scholar] [CrossRef]
- Machado, R.C.L.; Alexis, F.; De Sousa, F.B.; Sousa, D. Nanostructured and photochromic material for environmental detection of metal ions. Molecules 2019, 24, 4243. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Lu, M.; Huang, X.; Li, T.; Xu, D. Application of gold-nanoparticle colorimetric sensing to rapid food safety screening. Sensors 2018, 18, 4166. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Huang, S.; Liu, Y.; He, S.; Zhao, L.; Zeng, X. Highly selective and sensitive fluorescent turn-on chemosensor for Al3+ based on a novel photoinduced electron transfer approach. Org. Lett. 2011, 13, 5274–5277. [Google Scholar] [CrossRef] [PubMed]
- Khansili, N.; Rattu, G.; Krishna, P.M. Label-free optical biosensors for food and biological sensor applications. Sens. Actuators B Chem. 2018, 265, 35–49. [Google Scholar] [CrossRef]
- Jiménez-López, J.; Ortega-Barrales, P.; Ruiz-Medina, A. Development of an semi-automatic and sensitive photochemically induced fluorescence sensor for the determination of thiamethoxam in vegetables. Talanta 2016, 149, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-J.; Peng, Y.-L.; Zhang, C.-G.; Li, Y.-B.; Liu, C.; And, Y.-B.L. Synthesis of a series of zinc porphyrins and spectroscopic changes upon coordination reaction with imidazole derivatives. Bull. Korean Chem. Soc. 2015, 36, 2693–2702. [Google Scholar] [CrossRef]
- Liu, Q.; Dong, J.; Sun, Q.; Zhao, S.; Chen, Y.; Jiang, J. A novel calix[4]arene-modified porphyrin-based dual-mode sensor for the specific detection of dopamine with excellent performance. New J. Chem. 2019, 43, 10376–10381. [Google Scholar] [CrossRef]
- Drouet, S.; Merhi, A.; Yao, D.; Cifuentes, M.P.; Humphrey, M.G.; Wielgus, M.; Olesiak-Banska, J.; Matczyszyn, K.; Samoć, M.; Paul, F.; et al. Cubic nonlinear optical properties of new zinc tetraphenyl porphyrins peripherally functionalized with electron-rich Ru(II) alkynyl substituents. Tetrahedron 2012, 68, 10351–10359. [Google Scholar] [CrossRef]
- Gungor, S.A.; Kose, M.; Tumer, F.; Tumer, M. Photoluminescence, electrochemical, SOD activity and selective chemosensor properties of novel asymmetric porphyrin–Schiff base compounds. Dyes Pigment. 2016, 130, 37–53. [Google Scholar] [CrossRef]
- Montaseri, H.; Kruger, C.; Abrahamse, H. Recent advances in porphyrin-based inorganic nanoparticles for cancer treatment. Int. J. Mol. Sci. 2020, 21, 3358. [Google Scholar] [CrossRef]
- Pucelik, B.; Sułek, A.; Drozd, A.; Stochel, G.; Pereira, M.M.; Pinto, S.; Arnaut, L.G.; Dąbrowski, J.M. Enhanced cellular uptake and photodynamic effect with amphiphilic fluorinated porphyrins: The role of sulfoester groups and the nature of reactive oxygen species. Int. J. Mol. Sci. 2020, 21, 2786. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Ma, Q.; Hu, X.; Gao, Y.; Yan, X.; Qin, D.; Lua, X. Design of a novel naked-eye and turn-on fluorescence sensor based on the 5,10,15,20-(4-sulphonatophenyl) porphyrin (TPPS4)-Hg2+ system: Monitoring of glutathione (GSH) in real samples and DFT calculation. Sens. Actuators B Chem. 2018, 254, 475–482. [Google Scholar] [CrossRef]
- Muthukumar, P.; John, S.A. Gold nanoparticles decorated on cobalt porphyrin-modified glassy carbon electrode for the sensitive determination of nitrite ion. J. Colloid Interface Sci. 2014, 421, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Masih, D.; Chernikova, V.; Shekhah, O.; Eddaoudi, M.; Mohammed, O.F. Zeolite-like metal–organic framework (MOF) encaged Pt(II)-porphyrin for anion-selective sensing. ACS Appl. Mater. Interfaces 2018, 10, 11399–11405. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, O.; Alcay, Y.; Kaya, K.; Sezen, M.; Atasen, S.K.; Yildirim, M.S.; Ozkilic, Y.; Tu¨zün, N.Ş.; Yilmaz, I. Superior sensor for Be2+ ion recognition via the unprecedented octahedral crystal structure of a one-dimensional coordination polymer of crown Fused Zinc Phthalocyanine. Inorg. Chem. 2018, 58, 909–923. [Google Scholar] [CrossRef]
- Ishida, M.; Naruta, Y.; Tani, F. A porphyrin-related macrocycle with an embedded 1,10-phenanthroline moiety: Fluorescent magnesium(II) ion sensor. Angew. Chem. Int. Ed. 2009, 49, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Wang, Y.; Zhang, S.; Huang, L.; Wang, S.-A.; Hua, D. Determination of trace uranyl ion by thermoresponsive porphyrin–terminated polymeric sensor. Talanta 2015, 131, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Paolesse, R.; Nardis, S.; Monti, D.; Stefanelli, M.; Di Natale, C. Porphyrinoids for chemical sensor applications. Chem. Rev. 2016, 117, 2517–2583. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Hong, K.-I.; Jang, W.-D. Design and applications of molecular probes containing porphyrin derivatives. Coord. Chem. Rev. 2018, 354, 46–73. [Google Scholar] [CrossRef]
- Zhang, P.; Hu, J.; Liu, B.; Yang, J.; Hou, H. Recent advances in metalloporphyrins for environmental and energy applications. Chemosphere 2019, 219, 617–635. [Google Scholar] [CrossRef]
- Ding, Y.; Zhu, W.-H.; Xie, Y.-S. Development of ion chemosensors based on porphyrin analogues. Chem. Rev. 2016, 117, 2203–2256. [Google Scholar] [CrossRef]
- Zhu, M.; Zhou, Y.; Yang, L.; Li, L.; Qi, D.; Bai, M.; Chen, Y.; Du, H.; Bian, Y. Synergistic coupling of fluorescent “Turn-Off” with spectral overlap modulated FRET for ratiometric Ag+ sensor. Inorg. Chem. 2014, 53, 12186–12190. [Google Scholar] [CrossRef]
- Huang, W.-B.; Gu, W.; Huang, H.-X.; Wang, J.-B.; Shen, W.-X.; Lv, Y.-Y.; Shen, J. A porphyrin–basesd fluorescent probe for optical detection of toxic Cd2+ ion in aqueous solution and living cells. Dyes Pigment. 2017, 143, 427–435. [Google Scholar] [CrossRef]
- Namitha, P.P.; Saji, A.; Francis, S.; Rajith, L. Water soluble porphyrin for the fluorescent determination of cadmium ions. J. Fluoresc. 2020, 30, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.-Q.; Yue, F.; Zhong, Y.-R.; Ye, B.-H. A copper(II) ion-selective on−off-type fluoroionophore based on zinc porphyrin−dipyridylamino. Inorg. Chem. 2007, 46, 7749–7755. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.-K.; Zhang, L.-N.; Zhu, L.-N.; Zhang, R.; Li, X.-Z.; Kong, D. A water-soluble, cationic bis-porphyrin exhibiting a more sensitive fluorescent response to Cu2+ relative to its monomeric counterpart. Sens. Actuators B Chem. 2017, 247, 179–187. [Google Scholar] [CrossRef]
- Han, Z.-X.; Luo, H.-Y.; Zhang, X.-B.; Kong, R.-M.; Shen, G.-L.; Yu, R.-Q. A ratiometric chemosensor for fluorescent determination of Hg2+ based on a new porphyrin-quinoline dyad. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 72, 1084–1088. [Google Scholar] [CrossRef]
- Lv, Y.-Y.; Gu, W.; Wang, J.-B.; Huang, W.-B.; Shen, W.-X.; Sun, X.-Y. A ratiometric fluorescence chemodosimeter for detecting Hg2+ in aqueous solutions and living cells. Sens. Actuators B Chem. 2017, 246, 1017–1024. [Google Scholar] [CrossRef] [Green Version]
- Virk, T.S.; Kaur, P.; Singh, K. Selective and reversible recognition of Hg2+ ions by Tetrathia[22]porphyrin(2.1.2.1). Spectrochim. Acta Part A 2018, 205, 534–539. [Google Scholar] [CrossRef]
- Li, C.-Y.; Zhang, X.-B.; Qiao, L.; Zhao, Y.; He, C.-M.; Huan, S.-Y.; Lu, L.-M.; Jian, L.-X.; Shen, G.-L.; Yu, R.-Q. Naphthalimide−porphyrin hybrid based ratiometric bioimaging probe for Hg2+: Well-resolved emission spectra and unique specificity. Anal. Chem. 2009, 81, 9993–10001. [Google Scholar] [CrossRef]
- Feng, G.; Mi, H.; Fei, Q.; Shan, H.; Wang, B.; Xu, H.; Li, G.; Chen, F.; Huan, Y. A facile fluorescent chemosensor based on a water-soluble porphyrin for Mo6+ in aqueous solution. Spectrochim. Acta Part A 2016, 167, 122–126. [Google Scholar] [CrossRef]
- Zhang, D.; Zhu, M.; Zhao, L.; Zhang, J.; Wang, K.; Qi, D.; Zhou, Y.; Bian, Y.; Jiang, J. Ratiometric fluorescent detection of Pb2+by FRET-based phthalocyanine-porphyrin dyads. Inorg. Chem. 2017, 56, 14533–14539. [Google Scholar] [CrossRef]
- Qi, D.; Zhang, J.; Zhang, D.; Zhu, M.; Gong, L.; Su, C.; Lu, W.; Bian, Y.; Jiang, J. A phthalocyanine-porphyrin triad for ratiometric fluorescent detection of lead(II) ions. Dyes Pigment. 2020, 173, 107941. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, K. Azacrown[N,S,O]-modified porphyrin sensor for detection of Ag+, Pb2+, and Cu2+. Photochem. Photobiol. Sci. 2013, 12, 2001–2007. [Google Scholar] [CrossRef] [PubMed]
- Zamadar, M.; Orr, C.; Uherek, M. Water soluble cationic porphyrin sensor for detection of Hg2+, Pb2+, Cd2+, and Cu2+. J. Sens. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, K.; Fukuzumi, S. An Yttrium ion-selective fluorescence sensor based on metal ion-controlled photoinduced electron transfer in zinc porphyrin−quinone dyad. J. Am. Chem. Soc. 2004, 126, 13922–13923. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, Y.; Li, R.; Wu, D.; Xu, R.; Li, S.; Zhang, Y.; Ye, H.; Xin, Q. A porphyrin-based optical sensor membrane prepared by electrostatic self-assembled technique for online detection of cadmium(II). Chemosphere 2019, 238, 124552. [Google Scholar] [CrossRef]
- Zhao, L.; Li, M.; Liu, M.; Zhang, Y.; Wu, C.; Zhang, Y. Porphyrin-functionalized porous polysulfone membrane towards an optical sensor membrane for sorption and detection of cadmium(II). J. Hazard. Mater. 2016, 301, 233–241. [Google Scholar] [CrossRef]
- Czolk, R.; Reichert, J.; Ache, H. An optical sensor for the detection of Cd(II) ions. Sens. Actuators A Phys. 1991, 26, 439–441. [Google Scholar] [CrossRef]
- Luo, H.-Y.; Zhang, X.-B.; Jiang, J.-H.; Li, C.-Y.; Peng, J.; Shen, G.-L.; Yu, R.-Q. An optode sensor for Cu2+ with high selectivity based on porphyrin derivative appended with bipyridine. Anal. Sci. 2007, 23, 551–555. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-B.; Guo, C.-C.; Li, Z.-Z.; Shen, G.-L.; Yu, R.-Q. An optical fiber chemical sensor for mercury ions based on a porphyrin dimer. Anal. Chem. 2002, 74, 821–825. [Google Scholar] [CrossRef]
- Dolci, L.S.; Marzocchi, E.; Montalti, M.; Prodi, L.; Monti, D.; Di Natale, C.; D’Amico, A.; Paolesse, R. Amphiphilic porphyrin film on glass as a simple and selective solid-state chemosensor for aqueous Hg2+. Biosens. Bioelectron. 2006, 22, 399–404. [Google Scholar] [CrossRef]
- Fang, Z.; Liu, B. A cationic porphyrin-based self-assembled film for mercury ion detection. Tetrahedron Lett. 2008, 49, 2311–2315. [Google Scholar] [CrossRef]
- Boroujerdi, R. Ce (III)—Porphyrin Sandwich Complex Ce2(TPP)3: A rod-like nanoparticle as a fluorescence turn-off probe for detection of Hg (II) and Cu (II). J. Fluoresc. 2016, 26, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Y.; Zhao, X.; Liu, B.; Xu, Y.; Wang, Y. A gadolinium(III)-porphyrin based coordination polymer for colorimetric and fluorometric dual mode determination of ferric ions. Microchim. Acta 2019, 186, 63. [Google Scholar] [CrossRef] [PubMed]
- Moradi, E.; Rahimi, R.; Farahani, Y.D.; Safarifard, V. Porphyrinic zirconium-based MOF with exposed pyrrole Lewis base site as a luminescent sensor for highly selective sensing of Cd2+ and Br− ions and THF small molecule. J. Solid State Chem. 2020, 282, 121103. [Google Scholar] [CrossRef]
- Hibbard, H.A.J.; Burnley, M.J.; Rubin, H.N.; Miera, J.A.; Reynolds, M.M. Porphyrin-based metal-organic framework and polyvinylchloride composites for fluorescence sensing of divalent cadmium ions in water. Inorg. Chem. Commun. 2020, 115, 107861. [Google Scholar] [CrossRef]
- Li, L.; Shen, S.; Lin, R.; Bai, Y.; Liu, H. Rapid and specific luminescence sensing of Cu(II) ions with a porphyrinic metal–organic framework. Chem. Commun. 2017, 53, 9986–9989. [Google Scholar] [CrossRef]
- Chen, J.; Chen, H.; Wang, T.; Li, J.; Wang, J.; Lua, X. Copper ion fluorescent probe based on Zr-MOFs composite material. Anal. Chem. 2019, 91, 4331–4336. [Google Scholar] [CrossRef]
- Cheng, C.; Zhang, R.; Wang, J.; Zhang, Y.; Wen, C.; Tan, Y.; Yang, M. An ultrasensitive and selective fluorescent nanosensor based on porphyrinic metal–organic framework nanoparticles for Cu2+ detection. Analyst 2020, 145, 797–804. [Google Scholar] [CrossRef]
- Xu, Z.; Meng, Q.; Cao, Q.; Xiao, Y.; Liu, H.; Han, G.; Wei, S.; Yan, J.; Wu, L. Selective sensing of copper ions by mesoporous porphyrinic metal–organic frameworks nanoovals. Anal. Chem. 2020, 92, 2201–2206. [Google Scholar] [CrossRef]
- Chen, Y.-Z.; Jiang, H.-L. Porphyrinic metal–organic framework catalyzed heck-reaction: Fluorescence “Turn-On” sensing of Cu(II) ion. Chem. Mater. 2016, 28, 6698–6704. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Z.; Li, Y.; Zhuang, Q.; Zhao, W.; Gu, J. Porphyrinic MOFs for reversible fluorescent and colorimetric sensing of mercury(II) ions in aqueous phase. RSC Adv. 2016, 6, 69807–69814. [Google Scholar] [CrossRef]
- Moradi, E.; Rahimi, R.; Safarifard, V. Porphyrinic zirconium-based MOF with exposed pyrrole Lewis base site as an efficient fluorescence sensing for Hg2+ ions, DMF small molecule, and adsorption of Hg2+ ions from water solution. J. Solid State Chem. 2020, 286, 121277. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Guo, L.; Chen, G.; Kong, R.-M.; Qu, F.; Xia, L. Colorimetric detection of Hg(II) based on the gold amalgam-triggered reductase mimetic activity in aqueous solution by employing AuNP@MOF nanoparticles. Analyst 2020, 145, 1362–1367. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhao, L.; Bai, H.; Hong, W.; Li, C.; Shi, G. Chemically converted graphene induced molecular flattening of 5,10,15,20-tetrakis (1-methyl-4-pyridinio) porphyrin and its application for optical detection of cadmium(II) ions. J. Am. Chem. Soc. 2009, 131, 13490–13497. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Peng, D.; Liang, R.-P.; Qiu, J.-D. Nitrogen-doped graphene quantum dots as a new catalyst accelerating the coordination reaction between cadmium(II) and 5,10,15,20-tetrakis (1-methyl-4-pyridinio) porphyrin for cadmium(II) sensing. Anal. Chem. 2015, 87, 10894–10901. [Google Scholar] [CrossRef] [PubMed]
- Zare-Dorabei, R.; Rahimi, R.; Koohi, A.; Zargari, S. Preparation and characterization of a novel tetrakis(4-hydroxyphenyl)porphyrin–graphene oxide nanocomposite and application in an optical sensor and determination of mercury ions. RSC Adv. 2015, 5, 93310–93317. [Google Scholar] [CrossRef]
- Peng, D.; Zhang, L.; Liang, R.-P.; Qiu, J.-D. Rapid detection of mercury ions based on nitrogen-doped graphene quantum dots accelerating formation of manganese porphyrin. ACS Sens. 2018, 3, 1040–1047. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.-W.; Xiao, S.-J.; Peng, D.; Chen, J.-Q.; Liang, R.-P.; Jiang, J.; Qiu, J.-D. Fluorescent molybdenum oxide quantum dots and Hg(II) synergistically accelerate cobalt porphyrin formation: A new strategy for trace Hg(II) analysis. ACS Appl. Nano Mater. 2018, 1, 1484–1491. [Google Scholar] [CrossRef]
- Yin, W.; Dong, X.; Yu, J.; Pan, J.; Yao, Z.; Gu, Z.; Zhao, Y. MoS2-nanosheet-assisted coordination of metal ions with porphyrin for rapid detection and removal of cadmium ions in aqueous media. ACS Appl. Mater. Interfaces 2017, 9, 21362–21370. [Google Scholar] [CrossRef]
- Yan, Q.; Chen, Z.-H.; Xue, S.-F.; Han, X.-Y.; Lin, Z.-Y.; Zhang, S.; Shi, G.; Zhang, M. Lanthanide-doped nanoparticles encountering porphyrin hydrate: Boosting a dual-mode optical nanokit for Cu2+ sensing. Sens. Actuators B Chem. 2018, 268, 108–114. [Google Scholar] [CrossRef]
- Prabphal, J.; Vilaivan, T.; Praneenararat, T. Fabrication of a paper–based turn–off fluorescence sensor for Cu2+ ion from a pyridinium porphyrin. ChemistrySelect 2018, 3, 894–899. [Google Scholar] [CrossRef]
- Prabpal, J.; Vilaivan, T.; Praneenararat, T. Paper-based heavy metal sensors from the concise synthesis of an anionic porphyrin: A practical application of organic synthesis to environmental chemistry. J. Chem. Educ. 2017, 94, 1137–1142. [Google Scholar] [CrossRef]
- Pratiwi, R.; Nguyen, M.P.; Ibrahim, S.; Yoshioka, N.; Henry, C.S.; Tjahjono, D.H. A selective distance-based paper analytical device for copper(II) determination using a porphyrin derivative. Talanta 2017, 174, 493–499. [Google Scholar] [CrossRef]
- Cho, Y.; Lee, S.S.; Jung, J.H. Recyclable fluorimetric and colorimetric mercury-specific sensor using porphyrin-functionalized Au@SiO2 core/shell nanoparticles. Analyst 2010, 135, 1551–1555. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, Z.; Chen, Z.; Yuan, W.; Li, M. A fluorescent nanoprobe based on cellulose nanocrystals with porphyrin pendants for selective quantitative trace detection of Hg2+. New J. Chem. 2017, 41, 10272–10280. [Google Scholar] [CrossRef]
- Hu, Y.; Meng, L.; Lu, Q. “Fastening” porphyrin in highly cross-linked polyphosphazene hybrid nanoparticles: Powerful red fluorescent probe for detecting mercury ion. Langmuir 2014, 30, 4458–4464. [Google Scholar] [CrossRef]
- Xue, R.; Feng, L.; Wei, S.; Dong, X.; Wang, Q.; Yang, Y.; Liao, Y.; Wang, H. Al3+ enhanced room temperature phosphorescence of Pd-porphyrin resided in hybrid supramolecular gels and used for detection of trace Hg2+ ions. Talanta 2019, 194, 183–188. [Google Scholar] [CrossRef]
- Wen, B.; Xue, J.-Q.; Zhou, X.-J.; Wu, Q.-W.; Nie, J.-J.; Xu, J.-T.; Du, B.-Y. Highly selective and sensitive setection of Pb2+ in aqueous solution using tetra(4–pyridyl)porphyrin–functionalized thermosensitive ionic microgels. ACS Appl. Mater. Interfaces 2018, 10, 25706–25716. [Google Scholar] [CrossRef]
- Casimiro, T.; Pires, S.M.; Faustino, M.A.; Simões, M.M.; Neves, M.G.; Santos, H.; Capelo, J.L.; Mota, J.P.; Lodeiro, C.; Oliveira, E.D.J. New dual colorimetric/fluorimetric probes for Hg2+ detection & extraction based on mesoporous SBA-16 nanoparticles containing porphyrin or rhodamine chromophores. Dyes Pigment. 2019, 161, 427–437. [Google Scholar]
- Tao, S.; Li, X.; Wang, C.; Meng, C. A dual–emission amphiphile/dye modified mesoporous silica as fluorescent sensor for the detection of Fe3+, Cr3+ and Al3+. ChemistrySelect 2016, 1, 3208–3214. [Google Scholar] [CrossRef]
- Khani, R.; Ghiamati, E.; Boroujerdi, R.; Rezaeifard, A.; Zaryabi, M.H. A new and highly selective turn–on fluorescent sensor with fast response time for the monitoring of cadmium ions in cosmetic, and health product samples. Spectrochim. Acta A 2016, 163, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Sallam, G.; Shaban, S.Y.; Nassar, A.; El-Khouly, M.E. Water soluble porphyrin as optical sensor for the toxic heavy metal ions in an aqueous medium. Spectrochim. Acta Part A 2020, 118609. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.; Nath, M. Novel π-extended pyrrolo[1,2-a]pyrazinoporphyrins: Synthesis, photophysical properties and mercuric ion recognition. Dyes Pigment. 2018, 152, 161–170. [Google Scholar] [CrossRef]
- Moura, N.M.; Núñez, C.; Santos, S.M.; Faustino, M.A.; Cavaleiro, J.A.; Neves, M.G.; Capelo, J.L.; Lodeiro, C. Synthesis, spectroscopy studies, and theoretical calculations of new fluorescent probes based on pyrazole containing porphyrins for Zn(II), Cd(II), and Hg(II) optical detection. Inorg. Chem. 2014, 53, 6149–6158. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Wu, L.; Shen, W.; Wang, J.; Xuan, G.; Sun, X. A porphyrin-based chemosensor for colorimetric and fluorometric detection of cadmium(II) with high selectivity. J. Porphyr. Phthalocyanines 2015, 19, 769–774. [Google Scholar] [CrossRef]
- Santos, C.; Oliveira, E.D.J.; Menezes, J.C.J.M.D.S.; Barata, J.; Faustino, M.A.; Ferreira, V.F.; Cavaleiro, J.A.; Neves, M.G.; Lodeiro, C. New coumarin–corrole and–porphyrin conjugate multifunctional probes for anionic or cationic interactions: Synthesis, spectroscopy, and solid supported studies. Tetrahedron 2014, 70, 3361–3370. [Google Scholar] [CrossRef]
- Ghosh, M.; Ta, S.; Banerjee, M.; Mahiuddin, M.; Das, D. Exploring the scope of photo-induced electron transfer–chelation-enhanced fluorescence–fluorescence resonance energy transfer processes for recognition and discrimination of Zn2+, Cd2+, Hg2+, and Al3+ in a ratiometric manner: Application to sea fish analysis. ACS Omega 2018, 3, 4262–4275. [Google Scholar]
- Mizoshita, N.; Yamanaka, K.-I.; Hiroto, S.; Shinokubo, H.; Tani, T.; Inagaki, S. Energy and electron Transfer from fluorescent mesostructured organosilica framework to guest dyes. Langmuir 2012, 28, 3987–3994. [Google Scholar] [CrossRef]
- Chen, G.; Song, F.; Xiong, X.; Peng, X. Fluorescent nanosensors based on fluorescence resonance energy transfer (FRET). Ind. Eng. Chem. Res. 2013, 52, 11228–11245. [Google Scholar] [CrossRef]
- Clapp, A.R.; Medintz, I.L.; Uyeda, H.T.; Fisher, B.R.; Goldman, E.R.; Bawendi, M.G.; Mattoussi, H. Quantum dot-based multiplexed fluorescence resonance energy transfer. J. Am. Chem. Soc. 2005, 127, 18212–18221. [Google Scholar] [CrossRef]
- Chen, Y.; Wan, L.; Yu, X.; Li, W.; Bian, Y.; Jiang, J. Rational design and synthesis for versatile FRET ratiometric sensor for Hg2+ and Fe2+: A flexible 8-hydroxyquinoline benzoate linked Bodipy-porphyrin dyad. Org. Lett. 2011, 13, 5774–5777. [Google Scholar] [CrossRef] [PubMed]
- Mazloum-Ardakani, M.; Dehghani, H.; Jalayer, M.; Zare, H.R. Potentiometric determination of silver(I) by selective membrane electrode based on derivative of porphyrin. Anal. Sci. 2004, 20, 1667–1672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlascici, D.; Cosma, E.-F.; Pica, E.M.; Cosma, V.; Bizerea, O.; Mihailescu, G.; Olenic, L. Free base porphyrins as ionophores for heavy metal sensors. Sensors 2008, 8, 4995–5004. [Google Scholar] [CrossRef] [PubMed]
- Isildak, O.; Ozbek, O. Silver(I)–selective PVC membrane potentiometric sensor based on 5,10,15,20–tetra (4–pyridyl)–21H, 23H–porphine and potentiometric applications. J. Chem. Sci. 2020, 132. [Google Scholar] [CrossRef]
- Joon, N.K.; Barnsley, J.E.; Ding, R.; Lee, S.; Latonen, R.-M.; Bobacka, J.; Gordon, K.C.; Ogawa, T.; Lisak, G. Silver(I)-selective electrodes based on rare earth element double-decker porphyrins. Sens. Actuators B Chem. 2020, 305, 127311. [Google Scholar] [CrossRef]
- Plaschke, M.; Czolk, R.; Ache, H. Fluorimetric determination of mercury with a water-soluble porphyrin and porphyrin-doped sol-gel films. Anal. Chim. Acta 1995, 304, 107–113. [Google Scholar] [CrossRef]
- Yu, S.; Li, S.; Liu, Y.; Cui, S.; Shen, X. High-performance microporous polymer membranes prepared by interfacial polymerization for gas separation. J. Membr. Sci. 2019, 573, 425–438. [Google Scholar] [CrossRef]
- Olmos, J.M.; Laborda, E.; Ortuño, J.Á.; Molina, A. Characterization of inclusion complexes of organic ions with hydrophilic hosts by ion transfer voltammetry with solvent polymeric membranes. Talanta 2017, 164, 636–644. [Google Scholar] [CrossRef]
- Lu, J.J.; Zhu, Z.; Wang, W.; Liu, S. Coupling sodium dodecyl sulfate−capillary polyacrylamide gel electrophoresis with matrix-assisted laser desorption ionization time-of-flight mass spectrometry via a poly(tetrafluoroethylene) membrane. Anal. Chem. 2011, 83, 1784–1790. [Google Scholar] [CrossRef] [Green Version]
- Aly, K.I.; Sayed, M.M.; Mohamed, M.G.; Kuo, S.W.; Younis, O. A facile synthetic route and dual function of network luminescent porous polyester and copolyester containing porphyrin moiety for metal ions sensor and dyes adsorption. Microporous Mesoporous Mater. 2020, 298, 110063. [Google Scholar] [CrossRef]
- Czolk, R.; Reichert, J.; Ache, H.J. An optical sensor for the detection of heavy-metal-ions. Contam. Soil ’90 1990, 90, 835–836. [Google Scholar]
- Zhi, F.; Lua, X.; Yang, J.; Wang, X.; Shang, H.; Zhang, S.; Xue, Z. Selective anion sensing through a self-assembled monolayer of thiol-end-functionalized porphyrin. J. Phys. Chem. C 2009, 113, 13166–13172. [Google Scholar] [CrossRef]
- Walton, K.S.; Snurr, R.Q. Applicability of the BET method for determining surface areas of microporous metal−organic frameworks. J. Am. Chem. Soc. 2007, 129, 8552–8556. [Google Scholar] [CrossRef] [PubMed]
- Fei, H.; Cohen, S.M. Metalation of a thiocatechol-functionalized Zr(IV)-based metal–organic framework for selective C–H functionalization. J. Am. Chem. Soc. 2015, 137, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; McGuirk, C.M.; Ross, M.B.; Wang, S.; Chen, P.-C.; Xing, H.; Liu, Y.; Mirkin, C.A. General and direct method for preparing oligonucleotide-functionalized metal–organic framework nanoparticles. J. Am. Chem. Soc. 2017, 139, 9827–9830. [Google Scholar] [CrossRef] [Green Version]
- Mallick, A.; El-Zohry, A.M.; Shekhah, O.; Yin, J.; Jia, J.; Aggarwal, H.; Emwas, A.-H.; Mohammed, O.F.; Eddaoudi, M. Unprecedented ultralow detection limit of amines using a thiadiazole-functionalized Zr(IV)-based metal–organic framework. J. Am. Chem. Soc. 2019, 141, 7245–7249. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.-X.; Yin, X.-D.; Bo, X.-J.; Guo, L.-P. High performance electrocatalyst based on MIL–101(Cr)/reduced graphene oxide composite: Facile synthesis and electrochemical detections. ChemElectroChem 2018, 5, 2893–2901. [Google Scholar] [CrossRef]
- Yang, D.; Odoh, S.O.; Borycz, J.; Wang, T.C.; Farha, O.K.; Hupp, J.T.; Cramer, C.J.; Gagliardi, L.; Gates, B.C. Tuning Zr6 metal–organic framework (MOF) nodes as catalyst supports: Site densities and electron-donor properties influence molecular iridium complexes as ethylene conversion catalysts. ACS Catal. 2015, 6, 235–247. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Xue, H.; Wang, Y.; Zhao, P.; Zhu, D.; Jiang, M.; Zhao, X. Solvothermal metal metathesis on a metal–organic framework with constricted pores and the study of gas separation. ACS Appl. Mater. Interfaces 2015, 7, 25402–25412. [Google Scholar] [CrossRef]
- Motakef-Kazemi, N.; Shojaosadati, S.A.; Morsali, A. In situ synthesis of a drug-loaded MOF at room temperature. Microporous Mesoporous Mater. 2014, 186, 73–79. [Google Scholar] [CrossRef]
- Liu, T.-F.; Feng, D.; Chen, Y.-P.; Zou, L.; Bosch, M.; Yuan, S.; Wei, Z.; Fordham, S.; Wang, K.; Zhou, H.-C. Topology-guided design and syntheses of highly stable mesoporous porphyrinic zirconium metal–organic frameworks with high surface area. J. Am. Chem. Soc. 2014, 137, 413–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, D.; Liu, X.; Huang, R.; Qi, W.; Su, R.; He, Z. One-pot synthesis of mercapto functionalized Zr-MOFs for the enhanced removal of Hg2+ ions from water. Chem. Commun. 2019, 55, 6775–6778. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.; Geim, A.K.; Morozov, S.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Chaichi, A.; Wang, Y.; Gartia, M.R. Substrate engineered interconnected graphene electrodes with ultrahigh energy and power densities for energy storage applications. ACS Appl. Mater. Interfaces 2018, 10, 21235–21245. [Google Scholar] [CrossRef]
- Praveena, M.; Sai, T.P.; Dutta, R.; Ghosh, A.; Basu, J.K. Electrically tunable enhanced photoluminescence of semiconductor quantum dots on graphene. ACS Photonics 2017, 4, 1967–1973. [Google Scholar] [CrossRef]
- Wang, L.; Ma, J.; Liu, Z.; Yi, X.; Zhu, H.; Wang, G. In situ fabrication of bendable microscale hexagonal pyramids array vertical light emitting diodes with graphene as stretchable electrical interconnects. ACS Photonics 2014, 1, 421–429. [Google Scholar] [CrossRef]

















| Type | Materials | Characteristics | Metal Ions | LOD | Reference |
|---|---|---|---|---|---|
| Molecular porphyrin | BODIPY-porphyrin dyad | Proportional detection of Ag+ in aqueous solution and living cells | Ag+ | 2.0 × 10−7 M | Zhu et al. [30] |
| Porphyrin derivative | Neutral pH, low cytotoxicity, completed within 5 min, reversible | Cd2+ | 3.2 × 10−8 M | Huang et al. [31] | |
| THMPP | Highly photostable and water soluble | Cd2+ | 1.5 × 10−8 M. | Namitha et al. [32] | |
| Zinc porphyrin-dipyridylamino | Detection of Cu2+ in methanol solution, reversible | Cu2+ | 3.3 × 10−7 M | Weng et al. [33] | |
| Bis-TMPipEOPP | With a higher tendency to self-aggregate | Cu2+ | 8.8 × 10−9 M | Liu et al. [34] | |
| Porphyrin-quinoline dyad | In ethanol solution, pH 5.0–9.0, Hg2+ concentration in the range of 3 × 10−7–2 × 10−5 M | Hg2+ | 2.2 × 10−8 M | Han et al. [35] | |
| Thiourea derivative | Visualized under ultraviolet light, with membrane permeability and low toxicity, application to Hg2+ paper strips | Hg2+ | 1.8 × 10−8 M | Lv et al. [36] | |
| Tetrathia porphyrin | Colorimetric detection of Hg2+ in aqueous solution, high sensitivity | Hg2+ | 0.04 ppb | Virk et al. [37] | |
| Naphthalimide-porphyrin hybrid | Hg2+ concentration in the range of 1.0 × 10−7–5.0 × 10−5 M, reversible and fast (response time less than 2 min) | Hg2+ | 2.0 × 10−8 M | Li et al. [38] | |
| H2TEHPPS | High sensitivity and selectivity towards Mo6+ at pH 3.5 | Mo6+ | 1.5 μg L−1 | Feng et al. [39] | |
| H2Pc-α-ZnPor H2Pc-β-ZnPor | Pb2+concentration in the range of 0−4.0 μM, with dual-channel detection | Pb2+ | 3.4 × 10−9 M 2.2 × 10−8 M | Zhang et al. [40] | |
| H2Pc-β-(ZnPor)2 | With a good linear relationship to Pb2+ concentration in the range of 0–2.0 μM | Pb2+ | 0.86 ppb | Qi et al. [41] | |
| Azacrown [N,S,O]-modified porphyrin | Simultaneous detection of Ag+, Pb2+ and Cu2+, Cu2+ with dual-mode detecting potential | Ag+ | 7.8 × 10−7 M | Chen and Wang [42] | |
| Cu2+ | 7.6 × 10–13 M | ||||
| Pb2+ | 1.3 × 10–11 M | ||||
| Cationic porphyrin | Ultraviolet detection of Hg2+, Pb2+, Cd2+, and Cu2+ in neutral aqueous solution | Cd2+ Cu2+ Hg2+ Pb2+ | 5 × 10−7 M 1.0 × 10−6 M 5 × 10−7 M 5 × 10−7 M | Zamadar et al. [43] | |
| ZnP-CONH-Q | With strong fluorescence enhancement | Y3+ | - | Okamoto and Fukuzumi [44] | |
| Porphyrin film | PAN/TMPyP/(PAH-PSS)3 membrane | Online visual and spectrophotometric sensing for cadmium during a flow-through process as well as static detection | Cd2+ | 1 ppm | Zhao et al. [45] |
| PSF-PNaSS/TMPyP film | Visual and spectrophotometric detection and adsorption to remove Cd2+ ions, adjustable adsorption capacity, sensitivity, response time, and detection limit, the sensor membrane has good stability and reusability | Cd2+ | − | Zhao et al. [46] | |
| TPPS/PVC membrane | Under alkaline conditions, the detection of Cd2+ within 20 min, and reversible | Cd2+ | 1.9 × 10–5 M | Czolk et al. [47] | |
| H2TPPBPy/PVC membrane | Cu2+ concentration in the range of 2.0 × 10–8−1.0 × 10–5 M, pH 6−8, reversible and fast (response time less than 5 min) | Cu2+ | 5 × 10−9 M | Luo et al. [48] | |
| DTPP/PVC membrane | Hg2+concentration in the range of 5.2 × 10−7−3.1 × 10−4 M, pH 2.4−8.0 | Hg2+ | 5.2 × 10−7 M | Zhang et al. [49] | |
| Amphiphilic porphyrin film | Water-soluble and renewable | Hg2+ | 1 × 10−5 M | Dolci et al. [50] | |
| Self-assembled film | Hg2+ concentration in the range of 1 × 10–10–1 × 10–6 M and with high stability | Hg2+ | 1 × 10–10 M | Fang and Liu [51] | |
| Metal complex | Ce2(TPP)3 | Detection of Hg2+ and Cu2+ ions in aqueous solution with color change | Cu2+ Hg2+ | 2.34 × 10−6 M 1.6 × 10−10 M | Boroujerdi [52] |
| Gd-TCPP | Colorimetric and fluorescent dual-mode detection of Fe3+, good water solubility, and determination of Fe3+ in fetal bovine serum samples | Fe3+ | 9.8 × 10−8 M | Chen et al. [53] | |
| MOFs | PCN-224 | Detection of Cd2+, Br−, and THF within 1 min | Cd2+ | 2 × 10–9 M | Moradi et al. [54] |
| MOF-PVC composite | With fast and sensitive detection of Cd2+ ions | Cd2+ | 0.3 ppb | Hibbard et al. [55] | |
| MOF-525 | High sensitivity in the Cu2+ ion concentration range of 0.1–1.2 mg L−1 | Cu2+ | 6.7 × 10−8 M | Li et al. [56] | |
| UiO-66(OH)2@PCN-224 | With built-in correction effect | Cu2+ | 6.8 × 10−11 M | Chen et al. [57] | |
| MOF-525 NPs | Linear range of 1.0−250 nM, detection of Cu2+ ion in water samples and living cells | Cu2+ | 2.2 × 10–10 M | Cheng et al. [58] | |
| PCN-222 | The linear range of Cu2+ concentration is 0.4–13 μM, and the response time is less than 3 seconds | Cu2+ | 5.0 × 10–8 M | Xu et al. [59] | |
| PCN-222-Pd(II) | Fluorescence enhanced, detection of Cu2+ in complex environments | Cu2+ | 5.0 × 10–8 M | Chen and Jiang [60] | |
| PCN-224 | Response rate as rapid as 2 min | Hg2+ | 6.0 × 10–9 M | Yang et al. [61] | |
| PCN-221 | A quenching response of Hg2+ ions with a fast fluorescent response rate under <1 min. | Hg2+ | 1.0 × 10–8 M | Moradi et al. [62] | |
| AuNP@MOF | With fast response time, high sensitivity and selectivity | Hg2+ | 1.03 × 10–10 M | Wang et al. [63] | |
| Graphene materials | TMPyP/CCG | Water-soluble | Cd2+ | 2 × 10−6 M | Xu et al. [64] |
| TMPyP /NGQDs | Detection of Cd2+ within 2 min at 25 °C, pH 7.0 | Cd2+ | 8.8 × 10−8 M | Zhang et al. [65] | |
| GO–THPP | Hg2+ concentration in the range of 6.0 × 10−9−6.0 × 10−5 M, pH 7.5, detection within 210 s, and with renewable ability | Hg2+ | 3.2 × 10−9 M | Dorabei et al. [66] | |
| TMPyP/NGQDs | Detection of Hg2 + in pH 7.0 and phosphate buffer | Hg2+ | 3.2 × 10−10 M | Peng et al. [67] | |
| TMPyP/MoO3−x QDs | Shorting analysis time, high sensitivity, intracellular imaging | Hg2+ | 8 × 10−10 M | Zhang et al. [68] | |
| Other materials | MoS2@TMPyP | Molybdenum disulfide accelerating the formation of metal porphyrin | Cd2+ | 7.2 × 10−8 M | Yin et al. [69] |
| UCNPs/TPPS | Using smartphones with color scanning APP to identify color changes in the detection process | Cu2+ | 1.3 × 10−6 M | Yan et al. [70] | |
| Paper-immobilized TMPyP | Combined with handheld UV lamp and smartphone or compact camera | Cu2+ | 0.16 ppm | Prabphal et al. [71] | |
| TSPP immobilized on paper | Detection within 5 min at neutral pH | Cu2+ | 1 × 10−4 M | Prabpal et al. [72] | |
| TDMPzP/Microfluidic paper | Suitable for testing under acidic conditions, pH 2.0−4.0 | Cu2+ | 1 ppm | Pratiwi et al. [73] | |
| Porphyrin-functionalized Au@SiO2 | Colorimetric fluorescence detection of Hg2+, color change within 10 s, with renewable fluorescence intensity | Hg2+ | 1.2 ppb | Cho et al. [74] | |
| CNC-SA-COOC6TPP | Good dispersion of cellulose nanocrystals | Hg2+ | 5 × 10−8 M | Chen et al. [75] | |
| TPP−PZS | With a color change in acetone solution and as a test strip for rapid detection of Hg2+ | Hg2+ | 10 ppb | Hu et al. [76] | |
| Pd-TCPP/Supramolecular hydrogels | Hybrid gel bundle, small size, Hg2+ concentration in the range of 6 × 10–8−1 × 10–6 M | Hg2+ | 1.7 × 10–11 M | Xue et al. [77] | |
| TPyP5-MGs | Uniform distribution(radius about 189 nm), heat sensitive | Pb2+ | 5.9 × 10−9 M | Wen et al. [78] | |
| SBA-16@Porphyrin | Colorimetric and fluorescence detection of Hg2+, Pb2+ and Cu2+ | Cu2+ Hg2+ Pb2+ | 3.6 ppm 3.8 ppm 5.8 ppm | Marcelo et al. [79] | |
| Amphiphile/porphyrin modified mesoporous silica | Dual-emission material | Al3+ Cr3+ Fe3+ | 2 × 10−4 M 1 × 10−4 M 8 × 10−5 M | Tao et al. [80] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Z.-L.; Cheng, Y.-H.; Xu, Z.; Chen, M.-L. Recent Advances in Porphyrin-Based Materials for Metal Ions Detection. Int. J. Mol. Sci. 2020, 21, 5839. https://doi.org/10.3390/ijms21165839
Qi Z-L, Cheng Y-H, Xu Z, Chen M-L. Recent Advances in Porphyrin-Based Materials for Metal Ions Detection. International Journal of Molecular Sciences. 2020; 21(16):5839. https://doi.org/10.3390/ijms21165839
Chicago/Turabian StyleQi, Zhen-Li, Yun-Hui Cheng, Zhou Xu, and Mao-Long Chen. 2020. "Recent Advances in Porphyrin-Based Materials for Metal Ions Detection" International Journal of Molecular Sciences 21, no. 16: 5839. https://doi.org/10.3390/ijms21165839