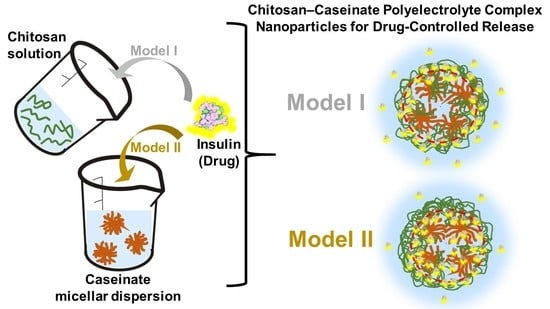
Nanoparticles and Colloidal Hydrogels of Chitosan–Caseinate Polyelectrolyte Complexes for Drug-Controlled Release Applications
Abstract
:1. Introduction
2. Results
2.1. Chitosans Macromolecular Structure and Thermal Properties
2.2. Spectroscopic Study of Chitosan/Caseinate Interaction
2.2.1. Fourier-Transform Infrared Spectroscopy (FTIR)
2.2.2. Raman Spectroscopy
2.3. Characterization of Chitosan-Caseinate Polyelectrolyte Complex Systems
2.3.1. Effect of Chitosan Molecular Weight
2.3.2. Effect of Chitosan Degree of Acetylation (DA)
2.3.3. Effect of Chitosan Molecular Weight
2.3.4. Effect of Chitosan Degree of Acetylation (DA)
2.4. Insulin Delivery Chitosan-Caseinate Nanoparticle Systems. Insulin Entrapment Efficiency and Release
3. Discussion
3.1. Effect of Chitosan Molecular Weight, Degree of Acetylation (DA) and Order of Addition of Polyelectrolytes on the Chitosan-Caseinate PEC Colloidal System Characteristics
3.2. Insulin Delivery from Chitosan-Caseinate Nanoparticles
4. Materials and Methods
4.1. Chitosan Degree of Acetylation (DA)
4.2. Chitosan Molecular Weight
4.3. Thermogravimetric Analysis TGA
4.4. Chitosan/Caseinate Interaction in Casted Films
Fourier Transform Infrared FTIR and Raman Spectroscopies
4.5. Preparation of Chitosan-Caseinate Nanoparticles
- (i)
- NaCas added to high molecular weight and low DA chitosan (HMW CHI)
- (ii)
- NaCas added to low molecular weight chitosan with low DA (deacetylated-LMW CHI)
- (iii)
- NaCas added to low molecular weight chitosan with high DA (LMW CHI)
- (iv)
- High molecular weight chitosan (HMW CHI) of low DA added to NaCas
- (v)
- Low molecular weight chitosan of low DA (deacetylated-LMW CHI) added to NaCas
- (vi)
- Low molecular weight chitosan (LMW CHI) of high DA added to NaCas
4.6. Characterization of Chitosan-Caseinate Polyelectrolyte Complexes
4.6.1. Zeta Potential and Hydrodynamic Radius
4.6.2. Morphology of Chitosan-Caseinate Nanoparticles
4.7. Insulin-Loaded Chitosan-Caseinate Nanoparticles for Drug Controlled Release
Insulin Entrapment Efficiency and Release Kinetics. UV Spectroscopy
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- E.U. Commission. Successful European Nanotechnology Research. Outstanding Science and Technology to Match the Needs of Future Society; EUR-OP: Luxembourg, 2011. [Google Scholar] [CrossRef]
- Kreuter, J. Nanoparticulate systems for brain delivery of drugs. Adv. Drug Deliv. Rev. 2001, 47, 65–81. [Google Scholar] [CrossRef]
- Mirza, A.Z.; Siddiqui, F.A. Nanomedicine and drug delivery: A mini review. Int. Nano Lett. 2014, 4, 94. [Google Scholar] [CrossRef] [Green Version]
- Jahangirian, H.; Lemraski, E.G.; Webster, T.J.; Rafiee-Moghaddam, R.; Abdollahi, Y. A review of drug delivery systems based on nanotechnology and green chemistry: Green nanomedicine. Int. J. Nanomed. 2017, 12, 2957–2978. [Google Scholar] [CrossRef] [Green Version]
- Meka, V.S.; Sing, M.K.; Pichika, M.R.; Nali, S.R.; Kolapalli, V.R.; Kesharwani, P. A comprehensive review on polyelectrolyte complexes. Drug Discov. Today 2017, 22, 1697–1706. [Google Scholar] [CrossRef]
- Kamdem Tamo, A.; Doench, I.; Morales Helguera, A.; Hoenders, D.; Walther, A.; Madrazo, A.O. Biodegradation of crystalline cellulose nanofibers by means of enzyme immobilized-alginate beads and microparticles. Polymers 2020, 12, 1522. [Google Scholar] [CrossRef]
- Homayun, B.; Lin, X.; Choi, H.-J. Challenges and recent progress in oral drug delivery systems for biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef] [Green Version]
- Abushammala, H.; Pontes, J.F.; Gomes, G.H.; Osorio-Madrazo, A.; Thiré, R.M.D.S.M.; Pereira, F.; Laborie, M.-P. Swelling, viscoelastic, and anatomical studies on ionic liquid-swollen Norway spruce as a screening tool toward ionosolv pulping. Holzforschung 2015, 69, 1059–1067. [Google Scholar] [CrossRef]
- Osorio-Madrazo, A.; Laborie, M.-P. Morphological and Thermal Investigations of Cellulosic Bionanocomposites. In Biopolymer Nanocomposites; Dufresne, A., Thomas, S., Pothen, L.A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 411–436. [Google Scholar]
- Mao, J.; Osorio-Madrazo, A.; Laborie, M.-P. Preparation of cellulose I nanowhiskers with a mildly acidic aqueous ionic liquid: Reaction efficiency and whiskers attributes. Cellulose 2013, 20, 1829–1840. [Google Scholar] [CrossRef]
- Samyn, P.; Osorio-Madrazo, A. Native Crystalline Polysaccharide Nanofibers: Processing and Properties. In Handbook of Nanofibers; Barhoum, A., Bechelany, M., Makhlouf, A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–36. [Google Scholar]
- García, D.E.; Glasser, W.G.; Pizzi, A.; Osorio-Madrazo, A.; Laborie, M.-P. Hydroxypropyl tannin derivatives from Pinus pinaster (Ait.) bark. Ind. Crop. Prod. 2013, 49, 730–739. [Google Scholar] [CrossRef]
- García, D.E.; Glasser, W.G.; Pizzi, T.A.; Osorio-Madrazo, A.; Laborie, M.-P. Synthesis and physicochemical properties of hydroxypropyl tannins from maritime pine bark (Pinus pinaster Ait.). Holzforschung 2014, 68, 411–418. [Google Scholar] [CrossRef]
- Osorio-Madrazo, A.; Eder, M.; Rueggeberg, M.; Pandey, J.K.; Harrington, M.J.; Nishiyama, Y.; Putaux, J.-L.; Rochas, C.; Burgert, I. Reorientation of cellulose nanowhiskers in agarose hydrogels under tensile loading. Biomacromolecules 2012, 13, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Osorio-Madrazo, A.; Laborie, M.-P. Novel preparation route for cellulose nanowhiskers. In Proceedings of the 245th American Chemical Society ACS Annual Meeting, New Orleans, LA, USA, 7–11 April 2013. [Google Scholar]
- Doench, I.; Torres-Ramos, M.E.W.; Montembault, A.; Oliveira, P.; Halimi, C.; Viguier, E.; Heux, L.; Siadous, R.; Thiré, R.M.D.S.M.; Madrazo, A.O. Injectable and gellable chitosan formulations filled with cellulose nanofibers for intervertebral disc tissue engineering. Polymers 2018, 10, 1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doench, I.; Tran, T.A.; David, L.; Montembault, A.; Viguier, E.; Gorzelanny, C.; Sudre, G.; Cachon, T.; Louback-Mohamed, M.; Horbelt, N.; et al. Cellulose nanofiber-reinforced chitosan hydrogel composites for intervertebral disc tissue repair. Biomimetics 2019, 4, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, M. Handbook of Encapsulation and Controlled Release. In Handbook of Encapsulation and Controlled Release; Mishra, M., Ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Osorio-Madrazo, A.; David, L.; Trombotto, S.; Lucas, J.-M.; Peniche-Covas, C.; Domard, A. Kinetics study of the solid-state acid hydrolysis of chitosan: Evolution of the crystallinity and macromolecular structure. Biomacromolecules 2010, 11, 1376–1386. [Google Scholar] [CrossRef]
- Osorio-Madrazo, A.; David, L.; Trombotto, S.; Lucas, J.-M.; Peniche-Covas, C.; Domard, A. Highly crystalline chitosan produced by multi-steps acid hydrolysis in the solid-state. Carbohydr. Polym. 2011, 83, 1730–1739. [Google Scholar] [CrossRef]
- Osorio-Madrazo, A.; David, L.; Peniche-Covas, C.; Rochas, C.; Putaux, J.-L.; Trombotto, S.; Alcouffe, P.; Domard, A. Fine microstructure of processed chitosan nanofibril networks preserving directional packing and high molecular weight. Carbohydr. Polym. 2015, 131, 1–8. [Google Scholar] [CrossRef]
- Chivrac, F.; Pollet, E.; Avérous, L. Progress in nano-biocomposites based on polysaccharides and nanoclays. Mater. Sci. Eng. R Rep. 2009, 67, 1–17. [Google Scholar] [CrossRef]
- Toeri, J.; Osorio-Madrazo, A.; Laborie, M.-P. Preparation and chemical/microstructural characterization of Azacrown ether-crosslinked chitosan films. Materials 2017, 10, 400. [Google Scholar] [CrossRef]
- Peniche Agüero, H.; David, L.; Peniche Covas, C.; Osorio-Madrazo, A. Bioinspired chitosan-BSA fibres for applications in intervertebral disc annulus fibrosus tissue engineering. Rev. Cuba. Investig. Biomédicas 2017, 36, 1–11. [Google Scholar]
- Peniche, H.; Osorio, A.; Acosta, N.; De La Campa, A.; Peniche, C. Preparation and characterization of superparamagnetic chitosan microspheres: Application as a support for the immobilization of tyrosinase. J. Appl. Polym. Sci. 2005, 98, 651–657. [Google Scholar] [CrossRef]
- Morganti, P.; Morganti, G. Chitin nanofibrils for advanced cosmeceuticals. Clin. Dermatol. 2008, 26, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Sarmentocde, B.; Ribeiro, A.J.; Veiga, F.; Ferreira, D. Development and characterization of new insulin containing polysaccharide nanoparticles. Colloids Surfaces B Biointerfaces 2006, 53, 193–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical applications of chitosan and its derivative nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janes, K.; Calvo, P.; Alonso, M.J. Polysaccharide colloidal particles as delivery systems for macromolecules. Adv. Drug Deliv. Rev. 2001, 47, 83–97. [Google Scholar] [CrossRef]
- Prabaharan, M.; Mano, J.F. Chitosan-based particles as controlled drug delivery systems. Drug Deliv. 2004, 12, 41–57. [Google Scholar] [CrossRef]
- Wu, D.; Zhu, L.; Li, Y.; Zhang, X.; Xu, S.; Yang, G.; Delair, T. Chitosan-based colloidal polyelectrolyte complexes for drug delivery: A review. Carbohydr. Polym. 2020, 238, 116126. [Google Scholar] [CrossRef]
- Drogoz, A.; David, L.; Rochas, C.; Domard, A.; Delair, T. Polyelectrolyte complexes from polysaccharides: Formation and stoichiometry monitoring. Langmuir 2007, 23, 10950–10958. [Google Scholar] [CrossRef]
- Wang, F.; Yang, Y.; Ju, X.; Udenigwe, C.C.; He, R. Polyelectrolyte complex nanoparticles from chitosan and acylated rapeseed cruciferin protein for curcumin delivery. J. Agric. Food Chem. 2018, 66, 2685–2693. [Google Scholar] [CrossRef]
- Lalevée, G.; Sudre, G.; Montembault, A.; Meadows, J.; Malaise, S.; Crépet, A.; David, L.; Delair, T. Polyelectrolyte complexes via desalting mixtures of hyaluronic acid and chitosan—Physicochemical study and structural analysis. Carbohydr. Polym. 2016, 154, 86–95. [Google Scholar] [CrossRef]
- Buschmann, M.D.; Merzouki, A.; Lavertu, M.; Thibault, M.; Jean, M.; Darras, V. Chitosans for delivery of nucleic acids. Adv. Drug Deliv. Rev. 2013, 65, 1234–1270. [Google Scholar] [CrossRef] [PubMed]
- De Kruif, C.G.; Huppertz, T.; Urban, V.S.; Petukhov, A.V. Casein micelles and their internal structure. Adv. Colloid Interface Sci. 2012, 171, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Głąb, T.; Boratynski, J. Potential of casein as a carrier for biologically active agents. Top. Curr. Chem. 2017, 375, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomar, P.; Nicolai, T.; Benyahia, L.; Durand, M. Comparative study of the rheology and the structure of sodium and calcium caseinate solutions. Int. Dairy J. 2013, 31, 100–106. [Google Scholar] [CrossRef]
- Thomar, P.; Nicolai, T. Dissociation of native casein micelles induced by sodium caseinate. Food Hydrocoll. 2015, 49, 224–231. [Google Scholar] [CrossRef]
- Coskun, A.E.I.; Sağlam, D.; Venema, P.; Van Der Linden, E.; Scholten, E. Preparation, structure and stability of sodium caseinate and gelatin micro-particles. Food Hydrocoll. 2015, 45, 291–300. [Google Scholar] [CrossRef]
- Morçöl, T.; Nagappan, P.; Nerenbaum, L.; Mitchell, A.; Bell, S. Calcium phosphate-PEG-insulin-casein (CAPIC) particles as oral delivery systems for insulin. Int. J. Pharm. 2004, 277, 91–97. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Mi, F.-L.; Chen, C.-T.; Chang, W.-C.; Peng, S.-F.; Liang, H.-F.; Sung, H.-W. Preparation and characterization of nanoparticles shelled with chitosan for oral insulin delivery. Biomacromolecules 2007, 8, 146–152. [Google Scholar] [CrossRef]
- Brange, J. Galenics of Insulin. The Physico-Chemical and Pharmaceutical Aspects of Insulin and Insulin Preparations; Springer: Berlin/Heidelberg, Germany, 1987. [Google Scholar] [CrossRef]
- Pan, Y.; Li, Y.-J.; Zhao, H.-Y.; Zheng, J.-M.; Xu, H.; Wei, G.; Hao, J.-S.; Cui, F.-D. Bioadhesive polysaccharide in protein delivery system: Chitosan nanoparticles improve the intestinal absorption of insulin in vivo. Int. J. Pharm. 2002, 249, 139–147. [Google Scholar] [CrossRef]
- Anal, A.K.; Tobiassen, A.; Flanagan, J.; Singh, H. Preparation and characterization of nanoparticles formed by chitosan–caseinate interactions. Colloids Surf. B Biointerfaces 2008, 64, 104–110. [Google Scholar] [CrossRef]
- Dhanasingh, S.; Nallaperumal, S.K. Chitosan/casein microparticles: Preparation, characterization and drug release studies. Int. J. Bioeng. Life Sci. World Acad. Sci. Eng. Technol. 2010, 4, 476–480. [Google Scholar]
- Nam, Y.S.; Park, W.H.; Ihm, D.; Hudson, S.M. Effect of the degree of deacetylation on the thermal decomposition of chitin and chitosan nanofibers. Carbohydr. Polym. 2010, 80, 291–295. [Google Scholar] [CrossRef]
- Iftime, M.M.; Marin, L. Chiral betulin-imino-chitosan hydrogels by dynamic covalent sonochemistry. Ultrason. Sonochem. 2018, 45, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Mauricio-Sánchez, R.A.; Salazar, R.; Luna-Bárcenas, J.G.; Mendoza-Galván, A. FTIR spectroscopy studies on the spontaneous neutralization of chitosan acetate films by moisture conditioning. Vib. Spectrosc. 2018, 94, 1–6. [Google Scholar] [CrossRef]
- Becerra, J.; Sudre, G.; Royaud, I.; Montserret, R.; Verrier, B.; Rochas, C.; Delair, T.; David, L. Tuning the hydrophilic/hydrophobic balance to control the structure of chitosan films and their protein release behavior. AAPS PharmSciTech 2016, 18, 1070–1083. [Google Scholar] [CrossRef]
- Zając, A.; Hanuza, J.; Wandas, M.; Dymińska, L. Determination of N-acetylation degree in chitosan using Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 114–120. [Google Scholar] [CrossRef]
- Dautzenberg, H. Polyelectrolyte complex formation in highly aggregating systems. 1. effect of salt: Polyelectrolyte complex formation in the presence of NaCl. Macromolecules 1997, 30, 7810–7815. [Google Scholar] [CrossRef]
- Müller, M.; Keßler, B.; Fröhlich, J.; Poeschla, S.; Torger, B. Polyelectrolyte complex nanoparticles of poly(ethyleneimine) and poly(acrylic acid): Preparation and applications. Polymers 2011, 3, 762–778. [Google Scholar] [CrossRef]
- Kabanov, V.A.; Zezin, A.B. A new class of complex water-soluble polyelectrolytes. Die Makromol. Chem. 1984, 6, 259–276. [Google Scholar] [CrossRef]
- Tsuchida, E.; Abe, K. Interactions Between Macromolecules in Solution and Intermacromolecular Complexes, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 45. [Google Scholar]
- Morris, G.A.; Sims, I.M.; Robertson, A.J.; Furneaux, R.H. Investigation into the physical and chemical properties of sodium caseinate-maltodextrin glyco-conjugates. Food Hydrocoll. 2004, 18, 1007–1014. [Google Scholar] [CrossRef] [Green Version]
- Dalgleish, D.; Spagnuolo, P.A.; Goff, H.D. A possible structure of the casein micelle based on high-resolution field-emission scanning electron microscopy. Int. Dairy J. 2004, 14, 1025–1031. [Google Scholar] [CrossRef]
- Calvo, P.; Remuñan-López, C.; Vila-Jato, J.L.; Alonso, M.J. Chitosan and chitosan/ethylene oxide-propylene oxide block copolymer nanoparticles as novel carriers for proteins and vaccines. Pharm. Res. 1997, 14, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Urrusuno, R.; Calvo, P.; Remuñán-López, C.; Vila-Jato, J.L.; Alonso, M.J. Enhancement of nasal absorption of insulin using chitosan nanoparticles. Pharm. Res. 1999, 16, 1576–1581. [Google Scholar] [CrossRef] [PubMed]
- Lamarque, G.; Cretenet, M.; Viton, C.; Domard, A. New route of deacetylation of α- and β-chitins by means of freeze−pump out−thaw cycles. Biomacromolecules 2005, 6, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Hirai, A.; Odani, H.; Nakajima, A. Determination of degree of deacetylation of chitosan by 1H NMR spectroscopy. Polym. Bull. 1991, 26, 87–94. [Google Scholar] [CrossRef]















| Type of Chitosan | Mn (g/mol) | Mw (g/mol) | PDI | DA (%) |
|---|---|---|---|---|
| HMW CHI | 4.10 × 105 ± 6.4% | 6.11 × 105 ± 9.6% | 1.49 ± 11.6% | 3 |
| LMW CHI | 3.87 × 105 ± 0.62% | 5.23 × 105 ± 0.73% | 1.34 ± 0.35% | 13 |
| deacetylated-LMW CHI | 7.52 × 104 ± 0.59% | 1.75 × 105 ± 0.31% | 2.327 ± 0.67% | 3 |
| Experiment | Chitosan Mw | Chitosan DA | Volume Ratio V(CHI):V(NaCas) |
|---|---|---|---|
| Case I: Chitosan in excess | |||
| (i) | High | Low | 50:1; - ; 10:1; - ; 5:1; 2:1; 1:1 |
| (ii) | Low | Low | 50:1; - ; 10:1; - ; 5:1; 2:1; 1:1 |
| (iii) | Low | High | 50:1; - ; 10:1; - ; 5:1; 2:1; 1:1 |
| Case II: Caseinate in excess | |||
| (iv) | High | Low | 1:50; 1:25; 1:10; 3:20; 1:5; 1:2; 1:1 |
| (v) | Low | Low | 1:50; 1:25; 1:10; 3:20; 1:5; 1:2; 1:1 |
| (vi) | Low | High | 1:50; 1:25; 1:10; 3:20; 1:5; 1:2; 1:1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lall, A.; Kamdem Tamo, A.; Doench, I.; David, L.; Nunes de Oliveira, P.; Gorzelanny, C.; Osorio-Madrazo, A. Nanoparticles and Colloidal Hydrogels of Chitosan–Caseinate Polyelectrolyte Complexes for Drug-Controlled Release Applications. Int. J. Mol. Sci. 2020, 21, 5602. https://doi.org/10.3390/ijms21165602
Lall A, Kamdem Tamo A, Doench I, David L, Nunes de Oliveira P, Gorzelanny C, Osorio-Madrazo A. Nanoparticles and Colloidal Hydrogels of Chitosan–Caseinate Polyelectrolyte Complexes for Drug-Controlled Release Applications. International Journal of Molecular Sciences. 2020; 21(16):5602. https://doi.org/10.3390/ijms21165602
Chicago/Turabian StyleLall, Aastha, Arnaud Kamdem Tamo, Ingo Doench, Laurent David, Paula Nunes de Oliveira, Christian Gorzelanny, and Anayancy Osorio-Madrazo. 2020. "Nanoparticles and Colloidal Hydrogels of Chitosan–Caseinate Polyelectrolyte Complexes for Drug-Controlled Release Applications" International Journal of Molecular Sciences 21, no. 16: 5602. https://doi.org/10.3390/ijms21165602