Oxidized Oligosaccharides Stabilize Rehydrated Sea Cucumbers against High-Temperature Impact
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation and Characterization of Oxidized Oligosaccharides
2.2. Texture Profile Analysis
- (1)
- Glutaraldehyde is less hydrophilic than oxidized sucrose. Protein hydrogel is a 3D network mainly stabilized by water-bridged hydrogen bondings. The fatty chain of glutaraldehyde is hydrophobic, so the introduction of this moiety to hydrogel is expected to break the proximal hydrogen bonding network and thus compromise the overall stabilizing effect. On the other hand, oxidized sucrose provides multiple hydrophilic groups that could serve as hydrogen bonding donors or acceptors and is thereby considered benign to the local hydrogen bonding network.
- (2)
- Glutaraldehyde has a shorter chain and fewer aldehydes per molecule than oxidized oligosaccharide does. The mechanism of crosslinking is imination between a crosslinker and at least two amines from different peptide chains (Figure 2A), which covalently connects the chains and thus stabilizes the hydrogel. This process is reasonably easy in solution, where molecules have high degree of freedom, yet is considerably harder in a gel system, because in the latter, the functional groups are “confined” to a relatively small space by various inter- and intra-molecular interactions and, as a result, cannot react as “freely” as they would in solution. For glutaraldehyde, whose reactivity in sea cucumber body wall is restricted by its short chain length, when no heteromolecular amine exists in the proximal, half-reacted glutaraldehyde may either form an intramolecular link with a proximal amine in the same protein or, in rarer cases, remain with the other end unreacted (Figure 2B, I→II), which both result in incomplete crosslinking. However, for oxidized sucrose, multiple aldehyde groups are located at different positions along a longer chain, which provided the accessibility and flexibility to react with both proximal and remote amine groups in the gel network. In addition, even if an intramolecular link should occur, since each oxidized sucrose bears four aldehyde groups, the remaining ones still may react intermolecularly, providing higher possibility for crosslinking (Figure 2B, I→III).
2.3. Degree of Imination and Total Soluble Material
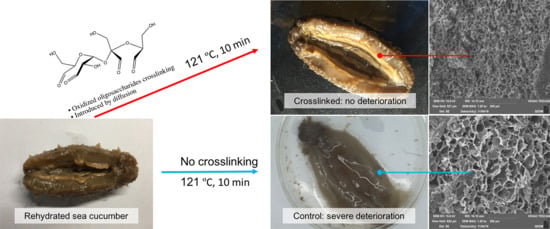
2.4. Appearance and SEM
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Oxidized Oligosaccharides
3.2.1. Synthesis of Normally Oxidized Oligosaccharides
3.2.2. Synthesis of Partially Oxidized Sucrose
3.2.3. Synthesis of Fully Oxidized Methylglucoside
3.3. Rehydration of Dry Sea Cucumber
3.4. Crosslinking of Rehydrated Sea Cucumber and High-Temperature Treatment
3.5. Texture Profile Analysis (TPA)
3.6. Aldehyde Content
3.7. Total Soluble Content
3.8. Degree of Amination
3.9. Ultrastructural Observation of Samples
3.10. Statistical Analysis
4. Conclusions and Future Work
5. Patents
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HTT | High-temperature treated |
| TLC | Thin Layer chromatography |
| TPA | Texture profile analysis |
| OPA | O-phthaldialdehyde |
| SDS | Sodium dodecyl sulfate |
| SEM | Scanning electron microscope |
| NMR | Nuclear magnetic resonance |
References
- Guo, J.; Zhang, Y.; Yang, X.Q. A novel enzyme cross-linked gelation method for preparing food globular protein-based transparent hydrogel. Food Hydrocoll. 2012, 26, 277–285. [Google Scholar] [CrossRef]
- Wang, H.; Shi, Y.; Wang, L.; Yang, Z. Recombinant proteins as cross-linkers for hydrogelations. Chem. Soc. Rev. 2013, 42, 891–901. [Google Scholar] [CrossRef]
- McKerchar, H.J.; Clerens, S.; Dobson, R.C.J.; Dyer, J.M.; Maes, E.; Gerrard, J.A. Protein-protein crosslinking in food: Proteomic characterisation methods, consequences and applications. Trends Food Sci. Technol. 2019, 86, 217–229. [Google Scholar] [CrossRef]
- Farjami, T.; Madadlou, A.; Labbafi, M. Characteristics of the bulk hydrogels made of the citric acid cross-linked whey protein microgels. Food Hydrocoll. 2015, 50, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Chattopadhyay, K.; Xavier, K.A.M.; Layana, P.; Balange, A.K.; Nayak, B.B. Chitosan hydrogel inclusion in fish mince based emulsion sausages: Effect of gel interaction on functional and physicochemical qualities. Int. J. Biol. Macromol. 2019, 134, 1063–1069. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Roohinejad, S.; George, S.; Barba, F.J.; Greiner, R.; Barbosa-Cánovas, G.V.; Mallikarjunan, K. Innovative food processing technologies on the transglutaminase functionality in protein-based food products: Trends, opportunities and drawbacks. Trends Food Sci. Technol. 2018, 75, 194–205. [Google Scholar] [CrossRef]
- Stevenson, M.; Long, J.; Seyfoddin, A.; Guerrero, P.; De la Caba, K.; Etxabide, A. Characterization of ribose-induced crosslinking extension in gelatin films. Food Hydrocoll. 2020, 99, 105324. [Google Scholar] [CrossRef]
- Hellwig, M.; Henle, T. Baking, Ageing, Diabetes: A Short History of the Maillard Reaction. Angew. Chem. Int. Ed. 2014, 53, 10316–10329. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, C.; Sun, X.; Zhang, S.; Yuan, Y.; Wang, D.; Xu, Y. Fabrication and characterization of cold-gelation whey protein-chitosan complex hydrogels for the controlled release of curcumin. Food Hydrocoll. 2020, 103, 105619. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, S.; Le, Y.; Qin, Z.; He, M.; Xu, F.; Zhu, Y.; Zhao, J.; Mao, C.; Zheng, L. An injectable collagen-genipin-carbon dot hydrogel combined with photodynamic therapy to enhance chondrogenesis. Biomaterials 2019, 218, 119190. [Google Scholar] [CrossRef]
- Beauchamp, R.O.; Clair, M.B.; Fennell, T.R.; Clarke, D.O.; Morgan, K.T.; Kair, F.W. A critical review of the toxicology of glutaraldehyde. Gastroenterol. Nurs. 1993, 16, 42–43. [Google Scholar] [CrossRef]
- El-Feky, G.S.; Zayed, G.M.; Elshaier, Y.A.M.M.; Alsharif, F.M. Chitosan-gelatin hydrogel crosslinked with oxidized sucrose for the ocular delivery of timolol maleate. J. Pharm. Sci. 2018, 107, 3098–3104. [Google Scholar] [CrossRef]
- Wang, P.; Sheng, F.; Tang, S.W.; Ud-Din, Z.; Chen, L.; Nawaz, A.; Hu, C.; Xiong, H. Synthesis and characterization of corn starch crosslinked with oxidized sucrose. Starch/Stärke 2019, 71, 1–8. [Google Scholar] [CrossRef]
- Kamimura, W.; Koyama, H.; Miyata, T.; Takato, T. Sugar-based crosslinker forms a stable atelocollagen hydrogel that is a favorable microenvironment for 3D cell culture. J. Biomed. Mater. Res. Part A 2014, 102, 4309–4316. [Google Scholar] [CrossRef]
- Mi, X.; Chang, Y.; Xu, H.; Yang, Y. Valorization of keratin from food wastes via crosslinking using non-toxic oligosaccharide derivatives. Food Chem. 2019, 300. [Google Scholar] [CrossRef]
- Xu, H.; Canisag, H.; Mu, B.; Yang, Y. Robust and flexible films from 100% starch cross-linked by biobased disaccharide derivative. ACS Sustain. Chem. Eng. 2015, 3, 2631–2639. [Google Scholar] [CrossRef]
- Liu, P.; Xu, H.; Mi, X.; Xu, L.; Yang, Y. Oxidized sucrose: A potent and biocompatible crosslinker for three-dimensional fibrous protein scaffolds. Macromol. Mater. Eng. 2015, 300, 414–422. [Google Scholar] [CrossRef]
- Xu, H.; Liu, P.; Mi, X.; Xu, L.; Yang, Y. Potent and regularizable crosslinking of ultrafine fibrous protein scaffolds for tissue engineering using a cytocompatible disaccharide derivative. J. Mater. Chem. B 2015, 3, 3609–3616. [Google Scholar] [CrossRef]
- Xu, D.; Su, L.; Zhao, P. Apostichopus japonicus in the worldwide production and trade of sea cucumbers. Dev. Aquac. Fish. Sci. 2015, 39, 383–398. [Google Scholar] [CrossRef]
- Cui, F.X.; Xue, C.H.; Li, Z.J.; Zhang, Y.Q.; Dong, P.; Fu, X.Y.; Gao, X. Characterization and subunit composition of collagen from the body wall of sea cucumber Stichopus japonicus. Food Chem. 2007, 100, 1120–1125. [Google Scholar] [CrossRef]
- Zhang, K.; Hou, H.; Bu, L.; Li, B.; Xue, C.; Peng, Z.; Su, S. Effects of heat treatment on the gel properties of the body wall of sea cucumber (Apostichopus japonicus). J. Food Sci. Technol. 2017, 54, 707–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.Y.; Xue, Y.; Dong, J.; Li, Z.J.; Wang, Y.M.; Xue, C.H. Effect of two plant extracts on the stability of the body wall collagen in instant sea cucumber. Mod. Food Sci. Technol. 2015, 31, 113–119. [Google Scholar] [CrossRef]
- Peng, Z.; Hou, H.; Feng, Y.L.; Cai, S.X.; Huang, M.; Zhang, Z.H.; Xue, C.H.; Zhao, X.; Li, B.F. Effects of phosphorylation and calcium cross-linking on the gel properties of sea cucumber collagen aggregates. Mod. Food Sci. Technol. 2015, 31, 190–195. [Google Scholar] [CrossRef]
- Maute, R.L.; Owens, M.L. Rapid determination of carbonyl content in acrylonitrile. Anal. Chem. 1956, 28, 1312–1314. [Google Scholar] [CrossRef]
- Pavlov, G.M.; Korneeva, E.V.; Smolina, N.A.; Schubert, U.S. Hydrodynamic properties of cyclodextrin molecules in dilute solutions. Eur. Biophys. J. 2010, 39, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Poddar, N.K.; Ansari, Z.A.; Singh, R.K.B.; Movahedi, A.A.M.; Ahmad, F. Effect of oligosaccharides and their monosaccharide mixtures on the stability of proteins: A scaled particle study. J. Biomol. Struct. Dyn. 2010, 28, 331–341. [Google Scholar] [CrossRef]
- Jeon, C.; Höll, W.H. Chemical modification of chitosan and equilibrium study for mercury ion removal. Water Res. 2003, 37, 4770–4780. [Google Scholar] [CrossRef]
- Reddy, N.; Reddy, R.; Jiang, Q. Crosslinking biopolymers for biomedical applications. Trends Biotechnol. 2015, 33, 362–369. [Google Scholar] [CrossRef]
- Benson, J.R.; Hare, P.E. O-Phthalaldehyde: Fluorogenic detection of primary amines in the picomole range. Comparison with fluorescamine and ninhydrin. Proc. Natl. Acad. Sci. USA 1975, 72, 619–622. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.Y.; Haraguchi, T.; Inazawa, T.; Kajiwara, S.; Yuasa, H. Synthesis of a novel class of glycocluster with a cyclic α-(1→6)-octaglucoside as a scaffold and their binding abilities to concanavalin A. Carbohydr. Res. 2010, 345, 2124–2132. [Google Scholar] [CrossRef]
- Mochizuki, Y. Texture Profile Analysis. Curr. Protoc. Food Anal. Chem. 2001. [Google Scholar] [CrossRef]
- Fan, M.; Huang, Q.; Zhong, S.; Li, X.; Xiong, S.; Xie, J.; Yin, T.; Zhang, B.; Zhao, S. Gel properties of myofibrillar protein as affected by gelatinization and retrogradation behaviors of modified starches with different crosslinking and acetylation degrees. Food Hydrocoll. 2019, 96, 604–616. [Google Scholar] [CrossRef]



| Entry | Oxidized Oligosaccharide | Actualaldehyde Content (mmol/g) | Theoretical Aldehyde Content (mmol/g) |
|---|---|---|---|
| 1 | Sucrose a | 5.04 ± 0.162 | 5.88 |
| 2 | Sucrose b | 9.14 ± 0.049 | 12.99 |
| 3 | raffinose | 6.83 ± 0.029 | 12.05 |
| 4 | stachyose | 7.73 ± 0.123 | 12.16 |
| 5 | α-cyclodextrin | 10.08 ± 0.038 | 12.50 |
| 6 | β-cyclodextrin | 9.54 ± 0.116 | 12.50 |
| 7 | γ-cyclodextrin | 9.94 ± 0.166 | 12.50 |
| 8 | methyl glucoside | 12.31 ± 0.12 | 12.34 |
| 9 | Dialdehyde starch | 4.7 ± 0.17 | 12.50 |
| Entry | Crosslinker 1 | Hardness (N) | Chewiness (mJ) | Resilience (N) | Springiness (mm) |
|---|---|---|---|---|---|
| 1 | control | 20.28 ± 4.74 a | 15.56 ± 7.56 a | 10.76 ± 3.36 a | 1.39 ± 0.35 a |
| 2 | sucrose 2 | 36.38 ± 4.12 bcd | 60.21 ± 9.9 bcd | 23.55 ± 0.79 bcde | 2.55 ± 0.35 cd |
| 3 | sucrose 3 | 61.2 ± 5.28 f | 138.67 ± 32.15 e | 48 ± 5.57 f | 2.89 ± 0.56 df |
| 4 | raffinose | 45.3 ± 3.71 def | 94.34 ± 9.17 de | 31.15 ± 2.71 def | 3.04 ± 0.31 f |
| 5 | stachyose | 34.2 ± 11.28 bc | 52.04 ± 31.59 abc | 23.75 ± 9.31 bcd | 2.07 ± 0.42 b |
| 6 | α-cyclodextrin | 33 ± 5.58 bc | 41.17 ± 7.45 ab | 21.4 ± 4.3 bc | 1.93 ± 0.08 b |
| 7 | β-cyclodextrin | 44.35 ± 6.81 de | 80.24 ± 21.67 cd | 31.3 ± 6.53 cde | 2.54 ± 0.24 cd |
| 8 | γ-cyclodextrin | 43.5 ± 8.46 cde | 73.81 ± 14.55 bcd | 31.68 ± 7.16 cde | 2.35 ± 0.13 bc |
| 9 | dialdehyde starch | 29.88 ± 3.79 ab | 35.93 ± 7.6 ab | 18.9 ± 2.46 ab | 1.89 ± 0.21 b |
| 10 | glutaraldehyde | 44.1 ± 13.84 cde | 74.82 ± 34.94 bcd | 31.53 ± 12.5 bcde | 2.29 ± 0.33 bc |
| 11 | methyl glucoside | 58.25 ± 1.2 ef | 115.72 ± 28.97 de | 38.55 ± 7.85 ef | 2.99 ± 0.14 df |
| Entry | Crosslinker | Degree of Imination (%) | Total Soluble Material (%) |
|---|---|---|---|
| 1 | None (control) | - | 25.34 |
| 2 | oxidized sucrose | 2.796 ± 0.0024 * | 7.78 |
| 3 | oxidized raffinose | 2.023 ± 0.0005 * | 13.60 |
| 4 | Oxidized β-cyclodextrin | 1.011 ± 0.0025 * | 10.44 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Xu, Y.; Xia, T.; Xue, C.; Liu, L.; Chang, P.; Wang, D.; Sun, X. Oxidized Oligosaccharides Stabilize Rehydrated Sea Cucumbers against High-Temperature Impact. Int. J. Mol. Sci. 2020, 21, 5204. https://doi.org/10.3390/ijms21155204
Liu J, Xu Y, Xia T, Xue C, Liu L, Chang P, Wang D, Sun X. Oxidized Oligosaccharides Stabilize Rehydrated Sea Cucumbers against High-Temperature Impact. International Journal of Molecular Sciences. 2020; 21(15):5204. https://doi.org/10.3390/ijms21155204
Chicago/Turabian StyleLiu, Jingyi, Yanan Xu, Tianhang Xia, Changhu Xue, Li Liu, Pengtao Chang, Dongfeng Wang, and Xun Sun. 2020. "Oxidized Oligosaccharides Stabilize Rehydrated Sea Cucumbers against High-Temperature Impact" International Journal of Molecular Sciences 21, no. 15: 5204. https://doi.org/10.3390/ijms21155204