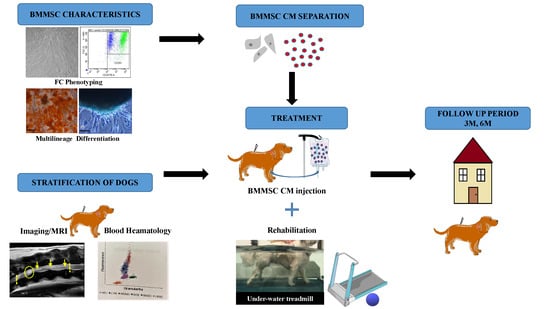
Stem Cell Conditioned Medium Treatment for Canine Spinal Cord Injury: Pilot Feasibility Study
Abstract
:1. Introduction
2. Results
2.1. Flow Cytometry with Canine CD Markers
2.2. Multilineage Potential
2.3. Locomotor and Bladder Function
2.4. Goniometry Measurements
2.5. Blood Analyses
3. Discussion
4. Materials and Methods
4.1. Canine BMMSC Characteristics and Conditioned Medium (CM)
4.1.1. Flow Cytometry with Canine BMMSC CD Markers
4.1.2. Three-Lineage Profile of BMMSC
4.1.3. BMMSC-Derived Conditioned Medium Preparation
4.2. Dogs Suffering from SCI
4.3. Clinical and Neurological Observation
4.4. Collection of Blood Samples
4.5. Locomotor Function
4.6. Imaging Methods-MRI
4.7. Treatment
4.8. Rehabilitation
4.9. Goniometric Assessment
4.10. Urination Assessment
- Complete loss of urinary function, manual expression needed at least three times daily, and it is hard to express the bladder manually;
- Partial recovery of urinary function, partial release of sphincter spasm, easier to express;
- Marked recovery of urinary function, release of sphincter spasm, easy to express, dog stretches tail and flexes legs when the bladder is manually expressed;
- Pronounced recovery of urinary function, marked release of sphincter spasm, very easy to express, dog sometimes urinates after irritation (tickle) of the perineum and seems nervous when the bladder is full;
- Complete recovery of urinary function, physiological urination outside the house, dog is always nervous with a full bladder.
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALB | Albumine |
| ALB/GLOB | Ratio albumine/globuline |
| ALKP | Alkaline phosphatase |
| ALT | Alanine aminotransferase |
| BMMSC | Bone marrow tissue-derived MSC |
| BMMSC CM | Bone marrow tissue-derived MSC conditioned medium |
| BDNF | Brain-derived growth factor |
| BUN/CREA | Ratio urea/creatinine |
| CBC | Complete blood count |
| CNS | Central nervous system |
| CM | Conditioned medium |
| CREA | Creatinine |
| CSF | Cerebrospinal fluid |
| DMEM | Dulbecco’s modification of Eagle’s medium |
| EDTA | Ethylene diamine tetra acetic acid |
| EV | Extracellular vesicles |
| FBS | Fetal bovine serum |
| GLOB | Globuline |
| GLU | Glucose |
| IVDD | Intervertebral disk |
| MRI | Magnetic resonance imaging |
| MPV | Mean platelet volume |
| MSC | Mesenchymal stromal cells |
| PBS | Phosphate-buffered saline |
| SCI | Spinal cord injury |
| TP | Total protein |
| UREA | Urea |
References
- Hou, S.; Rabchevsky, A.G. Autonomic consequences of spinal cord injury. Compr. Physiol. 2014, 4, 1419–1453. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Primer 2017, 3, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Svihra, J.; Krhut, J.; Zachoval, R.; Svihrova, V.; Luptak, J. Impact of clean intermittent catheterization on quality adjusted life years (QALYs) in spinal cord injury patients with neurogenic urinary incontinence. Neurourol. Urodyn. 2018, 37, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Batcher, K.; Dickinson, P.; Giuffrida, M.; Sturges, B.; Vernau, K.; Knipe, M.; Rasouliha, S.H.; Drögemüller, C.; Leeb, T.; Maciejczyk, K.; et al. Phenotypic Effects of FGF4 Retrogenes on Intervertebral Disc Disease in Dogs. Genes 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olby, N.; Griffith, E.; Levine, J.M. Comparison of Gait Assessment Scales in Dogs with Spinal Cord Injury due to Intervertebral Disc Herniation. J. Neurotrauma 2020. [Google Scholar] [CrossRef]
- Webb, A.A.; Ngan, S.; Fowler, D. Spinal cord injury II: Prognostic indicators, standards of care, and clinical trials. Can. Vet. J. 2010, 51, 598–604. [Google Scholar]
- Frank, L.R.; Roynard, P.F.P. Veterinary Neurologic Rehabilitation: The Rationale for a Comprehensive Approach. Top. Companion Anim. Med. 2018, 33, 49–57. [Google Scholar] [CrossRef]
- Lewis, M.J.; Howard, J.F.; Olby, N.J. The Relationship between Trans-Lesional Conduction, Motor Neuron Pool Excitability, and Motor Function in Dogs with Incomplete Recovery from Severe Spinal Cord Injury. J. Neurotrauma 2017, 34, 2994–3002. [Google Scholar] [CrossRef]
- Cizkova, D.; Le Marrec-Croq, F.; Franck, J.; Slovinska, L.; Grulova, I.; Devaux, S.; Lefebvre, C.; Fournier, I.; Salzet, M. Alterations of protein composition along the rostro-caudal axis after spinal cord injury: Proteomic, in vitro and in vivo analyses. Front. Cell. Neurosci. 2014, 8, 105. [Google Scholar] [CrossRef] [Green Version]
- Bracken, M.B.; Shepard, M.J.; Collins, W.F.; Holford, T.R.; Young, W.; Baskin, D.S.; Eisenberg, H.M.; Flamm, E.; Leo-Summers, L.; Maroon, J. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N. Engl. J. Med. 1990, 322, 1405–1411. [Google Scholar] [CrossRef]
- Cheung, V.; Hoshide, R.; Bansal, V.; Kasper, E.; Chen, C.C. Methylprednisolone in the management of spinal cord injuries: Lessons from randomized, controlled trials. Surg. Neurol. Int. 2015, 6. [Google Scholar] [CrossRef]
- Pêgo, A.P.; Kubinova, S.; Cizkova, D.; Vanicky, I.; Mar, F.M.; Sousa, M.M.; Sykova, E. Regenerative medicine for the treatment of spinal cord injury: More than just promises? J. Cell. Mol. Med. 2012, 16, 2564–2582. [Google Scholar] [CrossRef] [PubMed]
- Cizkova, D.; Novotna, I.; Slovinska, L.; Vanicky, I.; Jergova, S.; Rosocha, J.; Radonak, J. Repetitive intrathecal catheter delivery of bone marrow mesenchymal stromal cells improves functional recovery in a rat model of contusive spinal cord injury. J. Neurotrauma 2011, 28, 1951–1961. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Sasaki, M.; Kataoka-Sasaki, Y.; Nakazaki, M.; Nagahama, H.; Oka, S.; Oshigiri, T.; Takebayashi, T.; Yamashita, T.; Kocsis, J.D.; et al. Intravenous infusion of mesenchymal stem cells promotes functional recovery in a model of chronic spinal cord injury. Neuroscience 2016, 335, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Cizkova, D.; Cubinkova, V.; Smolek, T.; Murgoci, A.-N.; Danko, J.; Vdoviakova, K.; Humenik, F.; Cizek, M.; Quanico, J.; Fournier, I.; et al. Localized Intrathecal Delivery of Mesenchymal Stromal Cells Conditioned Medium Improves Functional Recovery in a Rat Model of Spinal Cord Injury. Int. J. Mol. Sci. 2018, 19, 870. [Google Scholar] [CrossRef] [Green Version]
- Cizkova, D.; Murgoci, A.-N.; Cubinkova, V.; Humenik, F.; Mojzisova, Z.; Maloveska, M.; Cizek, M.; Fournier, I.; Salzet, M. Spinal Cord Injury: Animal Models, Imaging Tools and the Treatment Strategies. Neurochem. Res. 2020, 45, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Cizkova, D.; Devaux, S.; Le Marrec-Croq, F.; Franck, J.; Slovinska, L.; Blasko, J.; Rosocha, J.; Spakova, T.; Lefebvre, C.; Fournier, I.; et al. Modulation properties of factors released by bone marrow stromal cells on activated microglia: An in vitro study. Sci. Rep. 2014, 4, 7514. [Google Scholar] [CrossRef] [Green Version]
- Ryu, H.-H.; Kang, B.-J.; Park, S.-S.; Kim, Y.; Sung, G.-J.; Woo, H.-M.; Kim, W.H.; Kweon, O.-K. Comparison of Mesenchymal Stem Cells Derived from Fat, Bone Marrow, Wharton’s Jelly, and Umbilical Cord Blood for Treating Spinal Cord Injuries in Dogs. J. Vet. Med. Sci. 2012, 14, 1617–1630. [Google Scholar] [CrossRef] [Green Version]
- Han, S.; Xiao, Z.; Li, X.; Zhao, H.; Wang, B.; Qiu, Z.; Li, Z.; Mei, X.; Xu, B.; Fan, C.; et al. Human placenta-derived mesenchymal stem cells loaded on linear ordered collagen scaffold improves functional recovery after completely transected spinal cord injury in canine. Sci. China Life Sci. 2018, 61, 2–13. [Google Scholar] [CrossRef]
- Carwardine, D.; Prager, J.; Neeves, J.; Muir, E.M.; Uney, J.; Granger, N.; Wong, L.-F. Transplantation of canine olfactory ensheathing cells producing chondroitinase ABC promotes chondroitin sulphate proteoglycan digestion and axonal sprouting following spinal cord injury. PLoS ONE 2017, 12, e0188967. [Google Scholar] [CrossRef] [Green Version]
- Granger, N.; Blamires, H.; Franklin, R.J.M.; Jeffery, N.D. Autologous olfactory mucosal cell transplants in clinical spinal cord injury: A randomized double-blinded trial in a canine translational model. Brain 2012, 135, 3227–3237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salazar, I.; Barrios Santos, W.A.; Zubizarreta, A.; Sánchez Quinteiro, P. Harvesting of olfactory ensheathing cells for autologous transplantation into the spinal cord injury. Its complexity in dogs. Front. Neuroanat. 2015, 9, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocsis, J.D.; Akiyama, Y.; Radtke, C. Neural precursors as a cell source to repair the demyelinated spinal cord. J. Neurotrauma 2004, 21, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Barnett, S.C.; Riddell, J.S. Olfactory ensheathing cells (OECs) and the treatment of CNS injury: Advantages and possible caveats. J. Anat. 2004, 204, 57–67. [Google Scholar] [CrossRef]
- Humenik, F.; Cizkova, D.; Cikos, S.; Luptakova, L.; Madari, A.; Mudronova, D.; Kuricova, M.; Farbakova, J.; Spirkova, A.; Petrovova, E.; et al. Canine bone marrow derived mesenchymal stem cells: Genomics, Proteomics and Functional Analyses of Paracrine Factor. Mol. Cell. Proteom. 2019, 18, 1824–1835. [Google Scholar] [CrossRef]
- Hejcl, A.; Sedý, J.; Kapcalová, M.; Toro, D.A.; Amemori, T.; Lesný, P.; Likavcanová-Mašínová, K.; Krumbholcová, E.; Prádný, M.; Michálek, J.; et al. HPMA-RGD hydrogels seeded with mesenchymal stem cells improve functional outcome in chronic spinal cord injury. Stem Cells Dev. 2010, 19, 1535–1546. [Google Scholar] [CrossRef]
- Forostyak, S.; Jendelova, P.; Sykova, E. The role of mesenchymal stromal cells in spinal cord injury, regenerative medicine and possible clinical applications. Biochimie 2013, 95, 2257–2270. [Google Scholar] [CrossRef]
- Lim, J.-H.; Byeon, Y.-E.; Ryu, H.-H.; Jeong, Y.-H.; Lee, Y.-W.; Kim, W.H.; Kang, K.-S.; Kweon, O.-K. Transplantation of canine umbilical cord blood-derived mesenchymal stem cells in experimentally induced spinal cord injured dogs. J. Vet. Sci. 2007, 8, 275. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.G.; Kang, Y.M.; Phi, J.H.; Kim, Y.-H.; Hwang, D.H.; Choi, J.Y.; Ryu, S.; Elastal, A.-E.; Paek, S.H.; Wang, K.-C.; et al. Implantation of polymer scaffolds seeded with neural stem cells in a canine spinal cord injury model. Cytotherapy 2010, 12, 841–845. [Google Scholar] [CrossRef]
- Bearden, R.N.; Huggins, S.S.; Cummings, K.J.; Smith, R.; Gregory, C.A.; Saunders, W.B. In-vitro characterization of canine multipotent stromal cells isolated from synovium, bone marrow, and adipose tissue: A donor-matched comparative study. Stem Cell Res. Ther. 2017, 8, 218. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Li, Q.; Luo, S.; Liu, Z.; Luo, D.; Zhang, B.; Zhang, D.; Rao, P.; Xiao, J. PPARγ and Wnt Signaling in Adipogenic and Osteogenic Differentiation of Mesenchymal Stem Cells. Curr. Stem Cell Res. Ther. 2016, 11, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G.; Prockop, D.J. Concise Review: Mesenchymal Stem/Multipotent Stromal Cells: The State of Transdifferentiation and Modes of Tissue Repair—Current Views. Stem Cells 2007, 25, 2896–2902. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, T.; Borjesson, D.L.; Osmond, C.; Griffon, D.J. Influence of Donor’s Age on Immunomodulatory Properties of Canine Adipose Tissue-Derived Mesenchymal Stem Cells. Stem Cells Dev. 2019, 28, 1562–1571. [Google Scholar] [CrossRef]
- Uccelli, A.; Benvenuto, F.; Laroni, A.; Giunti, D. Neuroprotective features of mesenchymal stem cells. Best Pract. Res. Clin. Haematol. 2011, 24, 59–64. [Google Scholar] [CrossRef]
- Chudickova, M.; Vackova, I.; Machova Urdzikova, L.; Jancova, P.; Kekulova, K.; Rehorova, M.; Turnovcova, K.; Jendelova, P.; Kubinova, S. The Effect of Wharton Jelly-Derived Mesenchymal Stromal Cells and Their Conditioned Media in the Treatment of a Rat Spinal Cord Injury. Int. J. Mol. Sci. 2019, 20, 4516. [Google Scholar] [CrossRef] [Green Version]
- Madrigal, M.; Rao, K.S.; Riordan, N.H. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J. Transl. Med. 2014, 12, 260. [Google Scholar] [CrossRef] [Green Version]
- Ren, K. Exosomes in perspective: A potential surrogate for stem cell therapy. Odontology 2019, 107, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Murgoci, A.-N.; Duhamel, M.; Raffo-Romero, A.; Mallah, K.; Aboulouard, S.; Lefebvre, C.; Kobeissy, F.; Fournier, I.; Zilkova, M.; Maderova, D.; et al. Location of neonatal microglia drives small extracellular vesicles content and biological functions in vitro. J. Extracell. Vesicles 2020, 9, 1727637. [Google Scholar] [CrossRef]
- Murgoci, A.-N.; Cizkova, D.; Majerova, P.; Petrovova, E.; Medvecky, L.; Fournier, I.; Salzet, M. Brain-Cortex Microglia-Derived Exosomes: Nanoparticles for Glioma Therapy. Chemphyschem Eur. J. Chem. Phys. Phys. Chem. 2018, 19, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhou, Y.; Zhang, R.; Wen, L.; Wu, K.; Li, Y.; Yao, Y.; Duan, R.; Jia, Y. Bone Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Recovery Following Spinal Cord Injury via Improvement of the Integrity of the Blood-Spinal Cord Barrier. Front. Neurosci. 2019, 13, 209. [Google Scholar] [CrossRef] [Green Version]
- Dray, C.; Rougon, G.; Debarbieux, F. Quantitative analysis by in vivo imaging of the dynamics of vascular and axonal networks in injured mouse spinal cord. Proc. Natl. Acad. Sci. USA 2009, 106, 9459–9464. [Google Scholar] [CrossRef] [Green Version]
- Marote, A.; Teixeira, F.G.; Mendes-Pinheiro, B.; Salgado, A.J. MSCs-Derived Exosomes: Cell-Secreted Nanovesicles with Regenerative Potential. Front. Pharmacol. 2016, 7, 231. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, Y.; Gong, F.; Rong, Y.; Luo, Y.; Tang, P.; Zhou, Z.; Zhou, Z.; Xu, T.; Jiang, T.; et al. Exosomes Derived from Bone Mesenchymal Stem Cells Repair Traumatic Spinal Cord Injury by Suppressing the Activation of A1 Neurotoxic Reactive Astrocytes. J. Neurotrauma 2019, 36, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jiao, G.; Wu, W.; Wang, H.; Ren, S.; Zhang, L.; Zhou, H.; Liu, H.; Chen, Y. Exosomes from Bone Marrow Mesenchymal Stem Cells Inhibit Neuronal Apoptosis and Promote Motor Function Recovery via the Wnt/β-catenin Signaling Pathway. Cell Transplant. 2019, 28, 1373–1383. [Google Scholar] [CrossRef]
- Anand, N.; Vaccaro, A.; Lim, M.; Lee, J.; Arnold, P.; Harrop, J.; Ratlif, J.; Rampersaud, R.; Bono, C. Evolution of Thoracolumbar Trauma Classification Systems: Assessing the Conflict Between Mechanism and Morphology of Injury. Top. Spinal Cord Inj. Rehabil. 2006, 12, 70–78. [Google Scholar] [CrossRef]
- Sims, C.; Waldron, R.; Marcellin-Little, D.J. Rehabilitation and Physical Therapy for the Neurologic Veterinary Patient. Vet. Clin. N. Am. Small Anim. Pract. 2015, 45, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Wang, C.; Wan, Z.; Lei, J. The possible mechanisms of massage therapy. Biomed. Res. 2019, 30, 6. [Google Scholar]
- Waters-Banker, C.; Dupont-Versteegden, E.E.; Kitzman, P.H.; Butterfield, T.A. Investigating the Mechanisms of Massage Efficacy: The Role of Mechanical Immunomodulation. J. Athl. Train. 2014, 49, 266–273. [Google Scholar] [CrossRef] [Green Version]
- Nadler, S.F.; Steiner, D.J.; Erasala, G.N.; Hengehold, D.A.; Hinkle, R.T.; Beth Goodale, M.; Abeln, S.B.; Weingand, K.W. Continuous Low-Level Heat Wrap Therapy Provides More Efficacy Than Ibuprofen and Acetaminophen for Acute Low Back Pain. Spine 2002, 27, 1012–1017. [Google Scholar] [CrossRef] [Green Version]
- Michlovitz, S.; Hun, L.; Erasala, G.N.; Hengehold, D.A.; Weingand, K.W. Continuous low-level heat wrap therapy is effective for treating wrist pain11A commercial party with a direct financial interest in the results of the research supporting this article has conferred or will confer a financial benefit on the author or/or more of the authors. Arch. Phys. Med. Rehabil. 2004, 85, 1409–1416. [Google Scholar] [CrossRef]
- Yan, Q.; Ruan, J.; Ding, Y.; Li, W.; Li, Y.; Zeng, Y. Electro-acupuncture promotes differentiation of mesenchymal stem cells, regeneration of nerve fibers and partial functional recovery after spinal cord injury. Exp. Toxicol. Pathol. 2011, 63, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, B.; Liu, T.; Zhao, D.; Hu, F. Effect of electroacupuncture on proliferation of astrocytes after spinal cord injury. Zhongguo Zhen Jiu. 2005, 25, 569–572. [Google Scholar] [PubMed]
- Cheng, P.-T.; Wong, M.-K.; Chang, P.-L. A therapeutic trial of acupuncture in neurogenic bladder of spinal cord injured patients—A preliminary report. Spinal Cord 1998, 36, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, W.; Wu, J.; Zhou, K.; Liu, B. Electroacupuncture Improves Bladder and Bowel Function in Patients with Traumatic Spinal Cord Injury: Results from a Prospective Observational Study. Available online: https://www.hindawi.com/journals/ecam/2013/543174/ (accessed on 22 May 2020).
- Hamid, S.; Hayek, R. Role of electrical stimulation for rehabilitation and regeneration after spinal cord injury: An overview. Eur. Spine J. 2008, 17, 1256–1269. [Google Scholar] [CrossRef] [Green Version]
- Ellapen, T.J.; Hammill, H.V.; Swanepoel, M.; Strydom, G.L. The benefits of hydrotherapy to patients with spinal cord injuries. Afr. J. Disabil. 2018, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Bose, P.K.; Hou, J.; Parmer, R.; Reier, P.J.; Thompson, F.J. Altered Patterns of Reflex Excitability, Balance, and Locomotion Following Spinal Cord Injury and Locomotor Training. Front. Physiol. 2012, 3, 258. [Google Scholar] [CrossRef] [Green Version]
- Teasell, R.; Bitensky, J.; Salter, K.; Bayona, N.A. The Role of Timing and Intensity of Rehabilitation Therapies. Top. Stroke Rehabil. 2005, 12, 46–57. [Google Scholar] [CrossRef]
- Aach, M.; Cruciger, O.; Sczesny-Kaiser, M.; Höffken, O.; Meindl, R.C.; Tegenthoff, M.; Schwenkreis, P.; Sankai, Y.; Schildhauer, T.A. Voluntary driven exoskeleton as a new tool for rehabilitation in chronic spinal cord injury: A pilot study. Spine J. 2014, 14, 2847–2853. [Google Scholar] [CrossRef]
- Nas, K.; Yazmalar, L.; Şah, V.; Aydın, A.; Öneş, K. Rehabilitation of spinal cord injuries. World J. Orthop. 2015, 6, 8–16. [Google Scholar] [CrossRef]
- Wang, D.; Ichiyama, R.M.; Zhao, R.; Andrews, M.R.; Fawcett, J.W. Chondroitinase Combined with Rehabilitation Promotes Recovery of Forelimb Function in Rats with Chronic Spinal Cord Injury. J. Neurosci. 2011, 31, 9332–9344. [Google Scholar] [CrossRef]
- Wang, L.; Conner, J.M.; Nagahara, A.H.; Tuszynski, M.H. Rehabilitation drives enhancement of neuronal structure in functionally relevant neuronal subsets. Proc. Natl. Acad. Sci. USA 2016, 113, 2750–2755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olby, N.J.; De Risio, L.; Muñana, K.R.; Wosar, M.A.; Skeen, T.M.; Sharp, N.J.; Keene, B.W. Development of a functional scoring system in dogs with acute spinal cord injuries. Am. J. Vet. Res. 2001, 62, 1624–1628. [Google Scholar] [CrossRef] [PubMed]
- Olby, N.; Harris, T.; Burr, J.; Muñana, K.; Sharp, N.; Keene, B. Recovery of pelvic limb function in dogs following acute intervertebral disc herniations. J. Neurotrauma 2004, 21, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Crook, T.; McGowan, C.; Pead, M. Effect of passive stretching on the range of motion of osteoarthritic joints in 10 labrador retrievers. Vet. Rec. 2007, 160, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Rees, K.; Prince, D.; Morehouse, J.; Shum-Siu, A.; Magnuson, D. Dynamic “Range of Motion” Hindlimb Stretching Disrupts Locomotor Function in Rats with Moderate Subacute Spinal Cord Injuries. J. Neurotrauma 2017, 34, 2086–2091. [Google Scholar] [CrossRef]
- Ribeiro, I.L.; Moreira, R.F.C.; Ferrari, A.V.; Alburquerque-Sendín, F.; Camargo, P.R.; Salvini, T.F. Effectiveness of early rehabilitation on range of motion, muscle strength and arm function after breast cancer surgery: A systematic review of randomized controlled trials. Clin. Rehabil. 2019, 33, 1876–1886. [Google Scholar] [CrossRef]
- Preston, T.; Wills, A.P. A single hydrotherapy session increases range of motion and stride length in Labrador retrievers diagnosed with elbow dysplasia. Vet. J. 2018, 234, 105–110. [Google Scholar] [CrossRef]
- Black, L.L.; Gaynor, J.; Gahring, D.; Adams, C.; Aron, D.; Harman, S.; Gingerich, D.A.; Harman, R. Effect of Adipose-Derived Mesenchymal Stem and Regenerative Cells on Lameness in Dogs with Chronic Osteoarthritis of the Coxofemoral Joints: A Randomized, Double-Blinded, Multicenter, Controlled Trial. Vet. Ther. 2007, 8, 13. [Google Scholar]
- Centeno, C.J.; Busse, D.; Kisiday, J.; Keohan, C.; Freeman, M.; Karli, D. Increased Knee Cartilage Volume in Degenerative Joint Disease using Percutaneously Implanted, Autologous Mesenchymal Stem Cells. Pain Physician 2008, 11, 343–353. [Google Scholar]
- Thomas, T.M.; Marcellin-Little, D.J.; Roe, S.C.; Lascelles, B.D.X.; Brosey, B.P. Comparison of measurements obtained by use of an electrogoniometer and a universal plastic goniometer for the assessment of joint motion in dogs. Am. J. Vet. Res. 2006, 67, 1974–1979. [Google Scholar] [CrossRef]
- Hady, L.L.; Fosgate, G.T.; Weh, J.M. Comparison of range of motion in Labrador Retrievers and Border Collies. J. Vet. Med. Anim. Health 2015, 7, 122–127. [Google Scholar] [CrossRef] [Green Version]
- Clemmons, R.M.; Bliss, E.L.; Dorsey-Lee, M.R.; Seachord, C.L.; Meyers, K.M. Platelet function, size and yield in whole blood and in platelet-rich plasma prepared using differing centrifugation force and time in domestic and food-producing animals. Thromb. Haemost. 1983, 50, 838–843. [Google Scholar] [CrossRef]
- Plasenzotti, R.; Stoiber, B.; Posch, M.; Windberger, U. Red blood cell deformability and aggregation behaviour in different animal species. Clin. Hemorheol. Microcirc. 2004, 31, 105–111. [Google Scholar] [PubMed]
- Millis, D.; Levine, D. Canine Rehabilitation and Physical Therapy; Elsevier Health Sciences: London, UK, 2013; p. 735. [Google Scholar]
- Jaegger, G.; Marcellin-Little, D.J.; Levine, D. Reliability of goniometry in Labrador Retrievers. Am. J. Vet. Res. 2002, 63, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Zink, M.C.; Van Dyke, J.B. Canine Sports Medicine and Rehabilitation; Wiley, J., Ed.; John Wiley and Sons Ltd.: Oxford, UK, 2013; pp. 82–99. [Google Scholar]






| A | ||||||
|---|---|---|---|---|---|---|
| Dog Z | Right Limb | Left Limb | Normal Ranges | |||
| Before Treatment | After Treatment | Before Treatment | After Treatment | |||
| Shoulder | flexion | 30 ± 1 | 40 ± 1.7 | 25 ± 1.7 | 30 ± 1.7 | 30–60 |
| extension | 140 ± 1.7 | 175 ± 2 | 165 ± 1 | 176 ± 1.7 | 160–170 | |
| Elbow | flexion | 35 ± 1.7 | 20 ± 1.7 | 30 ± 1 | 20 ± 2 | 20–40 |
| extension | 175 ± 1 | 155 ± 1 | 160 ± 1.7 | 170 ± 3.4 | 160–170 | |
| Carpus | flexion | 16 ± 1 | 15 ± 1 | 16 ± 1 | 14 ± 1 | 20–35 |
| extension | 220 ± 3.4 | 185 ± 1.7 | 220 ± 2.6 | 190 ± 1 | 190–200 | |
| Hip | flexion | 39 ± 2.6 | 39 ± 1 | 40 ± 1.7 | 40 ± 1 | 55 |
| extension | 174 ± 2 | 140 ± 1.7 | 170 ± 2 | 157 ± 1 | 160–165 | |
| Stifle | flexion | 20 ± 1 | 10 ± 1 | 25 ± 1.7 | 17 ± 1.7 | 45 |
| extension | 170 ± 1 | 175 ± 1 | 182 ± 1 | 168 ± 1 | 160–170 | |
| Tarsus | flexion | 25 ± 1 | 25 ± 1 | 15 ± 1 | 15 ± 1 | 40 |
| extension | 165 ± 1 | 190 ± 1 | 185 ± 1.7 | 185 ± 1 | 170 | |
| B | ||||||
| Dog B | Right Limb | Left Limb | Normal Ranges | |||
| Before Treatment | After Treatment | Before Treatment | After Treatment | |||
| Shoulder | flexion | 48 ± 1 | 55 ± 2.6 | 89 ± 1.7 | 45 ± 2 | 30–60 |
| extension | 137.5 ± 0.8 | 160 ± 2.6 | 178 ± 2 | 169 ± 1.3 | 160–170 | |
| Elbow | flexion | 26 ± 1 | 20 ± 2.6 | 25 ± 2 | 19 ± 1.7 | 20–40 |
| extension | 143 ± 1 | 162 ± 1.5 | 175 ± 1.7 | 163 ± 1.7 | 160–170 | |
| Carpus | flexion | 14 ± 1.7 | 16 ± 2 | 15 ± 1.7 | 16 ± 1 | 20–35 |
| extension | 163 ± 1 | 190 ± 2 | 175 ± 2 | 180 ± 2 | 190–200 | |
| Hip | flexion | 10 ± 2 | 10 ± 1.7 | 38 ± 1 | 32 ± 1 | 55 |
| extension | 145 ± 1 | 129 ± 2 | 96 ± 2 | 145 ± 2.6 | 160–165 | |
| Stifle | flexion | 22 ± 2.6 | 25 ± 2 | 40 ± 2 | 45 ± 1.7 | 45 |
| extension | 130 ± 1.7 | 145 ± 1.7 | 135 ± 1.7 | 155 ± 3 | 160–170 | |
| Tarsus | flexion | 30 ± 2 | 39 ± 2 | 20 ± 2.6 | 28 ± 1 | 40 |
| extension | 145 ± 1.7 | 153 ± 2 | 145 ± 1 | 168 ± 1.7 | 170 | |
| C | ||||||
| Dog M | Right Limb | Left Limb | Normal Ranges | |||
| Before Treatment | After Treatment | Before Treatment | After Treatment | |||
| Shoulder | flexion | 45 ± 1 | 35 ± 2 | 49 ± 1.7 | 35 ± 2 | 30–60 |
| extension | 185 ± 2 | 140 ± 1.7 | 200 ± 2 | 170 ± 2.6 | 160–170 | |
| Elbow | flexion | 35 ± 2 | 30 ± 1.7 | 28 ± 1 | 30 ± 1 | 20–40 |
| extension | 218 ± 1.7 | 184 ± 1.7 | 183 ± 2 | 210 ± 2 | 160–170 | |
| Carpus | flexion | 19 ± 2 | 20 ± 2 | 19 ± 2 | 19 ± 1 | 20–35 |
| extension | 168 ± 2 | 124 ± 1.7 | 210 ± 2 | 235 ± 2 | 190–200 | |
| Hip | flexion | 13 ± 1 | 10 ± 1 | 8 ± 1 | 20 ± 2 | 55 |
| extension | 187 ± 1 | 180 ± 1.7 | 169 ± 1 | 180 ± 2.6 | 160–165 | |
| Stifle | flexion | 19 ± 2 | 22 ± 1 | 10 ± 1.7 | 20 ± 1 | 45 |
| extension | 184 ± 1 | 170 ± 2 | 175 ± 2.6 | 130 ± 2 | 160–170 | |
| Tarsus | flexion | 35 ± 1.7 | 40 ± 1.7 | 25 ± 1.7 | 30 ± 1.7 | 40 |
| extension | 185 ± 1 | 185 ± 1.7 | 173 ± 2 | 190 ± 1.7 | 170 | |
| Hematological Parameters | Dog B | Dog M | Dog T | Dog Z | Normal Ranges | ||||
|---|---|---|---|---|---|---|---|---|---|
| BT | AT | BT | AT | BT | AT | BT | AT | ||
| RBC (×1012/L) | 5.97 | 8.09 | 7.53 | 7.97 | 8.75 | 6.27 | 6.24 | 7.35 | 5.65–8.87 |
| HCT (%) | 39.1 | 51.3 | 48.4 | 51.3 | 55.6 | 38.1 | 39.3 | 50.7 | 37.3–61.7 |
| HGB (g/DL) | 14.1 | 17.6 | 16.2 | 17.3 | 19 | 134 | 13.2 | 16.6 | 13.1–20.5 |
| MCV (fL) | 65.5 | 63.4 | 64.3 | 64.4 | 63.5 | 60.8 | 63 | 69 | 61.6–73.5 |
| MCH (pg) | 23.6 | 21.8 | 21.5 | 21.7 | 21.7 | 21.4 | 21.2 | 22.6 | 21.2–25.9 |
| MCHC (g/DL) | 36.1 | 34.3 | 33.5 | 33.7 | 34.2 | 35.2 | 33.6 | 32.7 | 32–37.9 |
| RDW (%) | 14.7 | 18.9 | 18.3 | 18.1 | 18.9 | 15.9 | 14.4 | 14.9 | 13.6–21.7 |
| %RETIC | 0.8 | 0.7 | 0.7 | 0.9 | 0.1 | 0.2 | 0.3 | 0.5 | |
| RETIC (K/µL) | 44.8 | 57.4 | 48.9 | 73.3 | 12.3 | 15 | 20.6 | 33.8 | 10–110 |
| RETIC-HGB (pg) | 27.6 | 25.9 | 24.4 | 22.4 | 25.6 | 23.6 | 241 | 25.5 | 22.3–29.6 |
| WBC (×10⁹/L) | 9.91 | 11.1 | 9.7 | 9.18 | 7.52 | 15.46 | 11.93 | 6.22 | 5.05–16.76 |
| %NEU | 67.7 | 74.7 | 71.1 | 71 | 72 | 72.2 | 75.5 | 65 | 60–77 |
| %LYM | 20.6 | 16.6 | 21.2 | 23.3 | 21.9 | 19.1 | 11.7 | 26.8 | 12–30 |
| %MONO | 6.6 | 4.4 | 6.8 | 3.5 | 3.3 | 5.9 | 4.4 | 5.5 | 3–10 |
| %EOS | 5.1 | 3.9 | 0.8 | 2.1 | 2.7 | 2.7 | 8.2 | 1.1 | 2–10 |
| %BASO | 0 | 0.4 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 1.6 | 0–1 |
| NEU (×10⁹/L) | 6.71 | 8.3 | 6.89 | 6.52 | 5.41 | 11.16 | 9.01 | 4.04 | 2.95–11.64 |
| LYM (×10⁹/L) | 2.04 | 1.84 | 2.06 | 2.14 | 1.65 | 2.95 | 1.39 | 1.67 | 1.05–5.10 |
| MONO (×10⁹/L) | 0.65 | 0.49 | 0.66 | 0.32 | 0.25 | 0.91 | 0.53 | 0.34 | 0.16–1.12 |
| EOS (×10⁹/L) | 0.1 | 0.43 | 0.08 | 0.19 | 0.2 | 0.42 | 0.98 | 0.07 | 0.06–1.23 |
| BASO (×10⁹/L) | 0 | 0.04 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.1 | 0.00–0.10 |
| PLT (K/µL) | 376 | 254 | 164 | 348 | 245 | 293 | 387 | 268 | 148–484 |
| MPV (fL) | 12 | 11.6 | 16.7 | 10 | 11.8 | 11.8 | 11.2 | 10.2 | 8.7–13.2 |
| PDW (fL) | 11.9 | 10 | not measured | 10.2 | 11.3 | 10.5 | 9.4 | 10.7 | 9.1–19.4 |
| PCT (%) | 0.45 | 0.9 | 0.27 | 0.35 | 0.29 | 0.35 | 0.43 | 0.3 | 0.14–0.46 |
| Breed | Dogs Name | Lesion Site | Age | Gender | Body Weight |
|---|---|---|---|---|---|
| Shi-tzu | Zuzi (Dog Z) | T11-L1 | 4 | Female | 5.4 kg |
| German Shepherd | Bak (Dog B) | T13-L2 | 6 | Male | 24 kg |
| Yorkshire Terrier | Max (Dog M) | T11-T12 | 5 | Male | 4 kg |
| Bichon | Tia (Dog T) | T13-L1 | 4 | Female | 6.4 kg |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vikartovska, Z.; Kuricova, M.; Farbakova, J.; Liptak, T.; Mudronova, D.; Humenik, F.; Madari, A.; Maloveska, M.; Sykova, E.; Cizkova, D. Stem Cell Conditioned Medium Treatment for Canine Spinal Cord Injury: Pilot Feasibility Study. Int. J. Mol. Sci. 2020, 21, 5129. https://doi.org/10.3390/ijms21145129
Vikartovska Z, Kuricova M, Farbakova J, Liptak T, Mudronova D, Humenik F, Madari A, Maloveska M, Sykova E, Cizkova D. Stem Cell Conditioned Medium Treatment for Canine Spinal Cord Injury: Pilot Feasibility Study. International Journal of Molecular Sciences. 2020; 21(14):5129. https://doi.org/10.3390/ijms21145129
Chicago/Turabian StyleVikartovska, Zuzana, Maria Kuricova, Jana Farbakova, Tomas Liptak, Dagmar Mudronova, Filip Humenik, Aladar Madari, Marcela Maloveska, Eva Sykova, and Dasa Cizkova. 2020. "Stem Cell Conditioned Medium Treatment for Canine Spinal Cord Injury: Pilot Feasibility Study" International Journal of Molecular Sciences 21, no. 14: 5129. https://doi.org/10.3390/ijms21145129