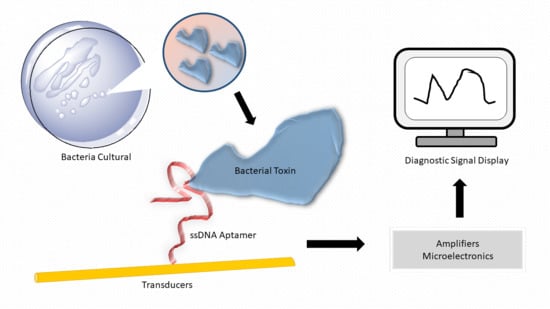
Recent Progress in the Identification of Aptamers Against Bacterial Origins and Their Diagnostic Applications
Abstract
:1. Introduction
2. In Vitro Selection of ssDNA Aptamers
2.1. Overview of SELEX Methodology for Aptamer Specific for Bacterial Related Targets
2.2. Highlights on Recent ssDNA Aptamers Specific to Bacterial Origins
2.2.1. Staphylococcus Aureus and Its Related Proteins
2.2.2. Pseudomonas aeruginosa
2.2.3. Mycobacterium Species and Related Proteins
2.2.4. Escherichia coli
2.2.5. Streptococcus Species
2.2.6. Bacillus Anthracis Virulence Factors
2.2.7. Vibrio Species
2.2.8. Helicobacter pylori
3. Diagnostic and Biosensing Applications of ssDNA Aptamer for Bacterial Infection
3.1. Overview of Common Detection Principles
3.2. Highlights of ssDNA Aptamer-Based Diagnostics for Bacterial Skin Infections
3.3. Highlights of ssDNA Aptamer-Based Diagnostics for Bacterial Respiratory System Infections
3.4. Highlights of ssDNA Aptamer-Based Diagnostics for Other Infections
4. Conclusions and Future Perspective
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MRE | Molecular recognition elements |
| LOD | Limit of detection |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| LPS | Lipopolysaccharides |
| SERS | Surface-enhanced Raman scattering |
| SPR | Surface plasmonresonance |
| FRET | Förster resonance energy transfer |
| SELEX | Systematic evolution of ligand by exponential enrichment |
| GO | Graphene oxide |
| AuNP | Gold nanoparticles |
| CFU | Colony forming unit |
| PCR | Polymerase chain reaction |
References
- Antibiotic Resistance Threats in the United States; Centres for Disease Control and Prevention: U.S. Department of Health and Human Services: Washington, DC, USA, 2013.
- Brouwer, M.C.; Tunkel, A.R.; van de Beek, D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin. Microbiol. Rev. 2010, 23, 467–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierre, D.M.; Baron, J.; Yu, V.L.; Stout, J.E. Diagnostic testing for Legionnaires’ disease. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 59. [Google Scholar] [CrossRef]
- Başpınar, E.Ö.; Dayan, S.; Bekçibaşı, M.; Tekin, R.; Ayaz, C.; Deveci, Ö.; Hoşoğlu, S. Comparison of culture and PCR methods in the diagnosis of bacterial meningitis. Braz J. Microbiol. 2017, 48, 232–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ustinova, V.V.; Smirnova, T.G.; Sochivko, D.G.; Varlamov, D.A.; Larionova, E.E.; Andreevskaya, S.N.; Andrievskaya, I.Y.; Kiseleva, E.A.; Chernousova, L.N.; Ergeshov, A. New assay to diagnose and differentiate between Mycobacterium tuberculosis complex and nontuberculous mycobacteria. Tuberculosis 2019, 114, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.L.; Sooter, L.J. Single-Stranded DNA Aptamers against Pathogens and Toxins: Identification and Biosensing Applications. Biomed. Res. Int. 2015, 2015, 419318. [Google Scholar] [CrossRef]
- Ali, M.H.; Elsherbiny, M.E.; Emara, M. Updates on Aptamer Research. Int. J. Mol. Sci. 2019, 20, 2511. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Mohamed, M.A.; Vinu Mohan, A.M.; Zhu, Z.; Sharma, V.; Mishra, G.K.; Mishra, R.K. Application of Electrochemical Aptasensors toward Clinical Diagnostics, Food, and Environmental Monitoring: Review. Sensors 2019, 19, 5435. [Google Scholar] [CrossRef] [Green Version]
- Hong, K.L.; Kaur Brar, S.; Hegde, K.; Pachapur, V.L. An overview of DNA/RNA-based monitoring tools and biosensors: Benefits and applications in the environmental toxicology. In Tools, Techniques and Protocols for Monitoring Environmental Contaminants; Elsevier, 2019; pp. 97–124. Available online: https://www.sciencedirect.com/science/article/pii/B9780128146798000054 (accessed on 20 June 2020).
- Pfeiffer, F.; Mayer, G. Selection and Biosensor Application of Aptamers for Small Molecules. Front. Chem. 2016, 4, 25. [Google Scholar] [CrossRef] [Green Version]
- Ruscito, A.; DeRosa, M.C. Small-Molecule Binding Aptamers: Selection Strategies, Characterization, and Applications. Front. Chem. 2016, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Hori, S.-I.; Herrera, A.; Rossi, J.J.; Zhou, J. Current Advances in Aptamers for Cancer Diagnosis and Therapy. Cancers 2018, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Potty, A.S.R.; Kourentzi, K.; Fang, H.; Schuck, P.; Willson, R.C. Biophysical characterization of DNA and RNA aptamer interactions with hen egg lysozyme. Int. J. Biol. Macromol. 2011, 48, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Elskens, J.P.; Elskens, J.M.; Madder, A. Chemical Modification of Aptamers for Increased Binding Affinity in Diagnostic Applications: Current Status and Future Prospects. Int. J. Mol. Sci. 2020, 21, 4522. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Raston, N.H.; Nguyen, V.T.; Gu, M.B. A new lateral flow strip assay (LFSA) using a pair of aptamers for the detection of Vaspin. Biosens. Bioelectron. 2017, 93, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, J.; Lee, B.H.; Song, C.S.; Gu, M.B. Specific detection of avian influenza H5N2 whole virus particles on lateral flow strips using a pair of sandwich-type aptamers. Biosens. Bioelectron. 2019, 134, 123–129. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Kwon, Y.S.; Gu, M.B. Aptamer-based environmental biosensors for small molecule contaminants. Curr. Opin. Biotechnol. 2017, 45, 15–23. [Google Scholar] [CrossRef]
- Takahashi, M. Aptamers targeting cell surface proteins. Biochimie 2018, 145, 63–72. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, M.; Su, H.; Xiao, H.; Wu, S.; Qin, X.; Li, S.; Mi, H.; Lu, Z.; Shi, D.; et al. Selection and characterization of ssDNA aptamers specifically recognizing pathogenic Vibrio alginolyticus. J. Fish. Dis. 2019, 42, 851–858. [Google Scholar] [CrossRef]
- Song, S.; Wang, X.; Xu, K.; Li, Q.; Ning, L.; Yang, X. Selection of highly specific aptamers to Vibrio parahaemolyticus using cell-SELEX powered by functionalized graphene oxide and rolling circle amplification. Anal. Chim. Acta 2019, 1052, 153–162. [Google Scholar] [CrossRef]
- Yan, W.; Gu, L.; Liu, S.; Ren, W.; Lyu, M.; Wang, S. Identification of a highly specific DNA aptamer for Vibrio vulnificus using systematic evolution of ligands by exponential enrichment coupled with asymmetric PCR. J. Fish. Dis. 2018, 41, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Masoum, A.; Oloomi, M.; Yavari, A.; Bouzari, S. DNA aptamer idenfitication and characterization for E. coli O157 detetion using cell based SELEX method. Anal. Biochem. 2017, 536, 36–44. [Google Scholar] [CrossRef]
- Zou, Y.; Duan, N.; Wu, S.; Shen, M.; Wang, Z. Selection, Identification, and Binding Mechanism Studies of an ssDNA Aptamer Targeted to Different Stages of E. coli O157:H7. J. Agric. Food Chem. 2018, 66, 5677–5682. [Google Scholar] [CrossRef] [PubMed]
- Marton, S.; Cleto, F.; Krieger, M.A.; Cardoso, J. Isolation of an Aptamer that Binds Specifically to E. coli. PLoS ONE 2016, 11, 0153637. [Google Scholar] [CrossRef] [Green Version]
- Renders, M.; Miller, E.; Lam, C.H.; Perrin, D.M. Whole cell-SELEX of aptamers with a tyrosine-like side chain against live bacteria. Org. Biomol. Chem. 2017, 15, 1980–1989. [Google Scholar] [CrossRef]
- Hamula, C.L.A.; Peng, H.; Wang, Z.; Tyrrell, G.J.; Li, X.-F.; Le, X.C. An improved SELEX technique for selection of DNA aptamers binding to M-type 11 of Streptococcus pyogenes. Methods 2016, 51–57. [Google Scholar] [CrossRef]
- Alfavian, H.; Mousavi Gargari, S.L.; Rasoulinejad, S.; Medhat, A. Development of a DNA aptamer that binds specifically to group A Streptococcus serotype M3. Can. J. Microbiol. 2017, 63, 160–168. [Google Scholar] [CrossRef]
- Cui, W.; Liu, J.; Su, D.; Hu, D.; Hou, S.; Hu, T.; Yang, J.; Luo, Y.; Xi, Q.; Chu, B.; et al. Identification of ssDNA aptamers specific to clinical isolates of Streptococcus mutans strains with different cariogenicity. Acta. Biochim. Et. Biophys. Sin. 2016, 48, 563–572. [Google Scholar] [CrossRef] [Green Version]
- Graziani, A.C.; Stets, M.I.; Lopes, A.L.K.; Schluga, P.H.C.; Marton, S.; Ferreira, I.M.; De Andrade, A.S.R.; Krieger, M.A.; Cardoso, J. High efficiency binding aptamers for a wide range of bacterial sepsis agents. J. Microbiol. Biotechnol. 2017, 27, 838–843. [Google Scholar] [CrossRef] [Green Version]
- Sypabekova, M.; Bekmurzayeva, A.; Wang, R.; Li, Y.; Nogues, C.; Kanayeva, D. Selection, Characterization, and application of DNA aptamers for detection of Mycobacterium tuberculosis secreted protein MPT64. Tuberculosis 2017, 104, 70–78. [Google Scholar] [CrossRef]
- Mozioglu, E.; Gokmen, O.; Tamerler, C.; Kocagoz, Z.T.; Akgoz, M. Selection of Nucleic Acid Aptamers Specific for Mycobacterium tuberculosis. Appl. Biochem. Biotechnol. 2016, 178, 849–864. [Google Scholar] [CrossRef]
- Sun, X.; Pan, Q.; Yuan, C.; Wang, Q.; Tang, X.L.; Ding, K.; Zhou, X.; Zhang, X.L. A Single ssDNA Aptamer Binding to Mannose-Capped Lipoarabinomannan of Bacillus Calmette-Guérin Enhances Immunoprotective Effect against Tuberculosis. J. Am. Chem. Soc. 2016, 138, 11680–11689. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.-L.; Wu, S.-m.; Xie, Y.S.; Neng, G.Q.; Yuan, C.; Zhou, X.; Zhang, X.-L. Generation and application of ssDNA aptamers against glycolipid antigen ManLAM of mycobacterium tuberculosis for TB diagnosis. J. Infect. 2016, 72, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Soundy, J.; Day, D. Selection of DNA aptamers specific for live Pseudomonas aeruginosa. PLoS ONE 2017, 12, e0185385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsao, S.M.; Lai, J.C.; Horng, H.E.; Liu, T.C.; Hong, C.Y. Generation of aptamers from A primer-free randomized ssDNA library using magnetic-assisted rapid aptamer selection. Sci. Rep. 2017, 7, 45478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, M.Y.; Nguyen, D.; Hong, S.W.; Kim, B.C. Broadly reactive aptamers targeting bacteria belonging to different genera using a sequential toggle cell-SELEX. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Lahousse, M.; Park, H.-C.; Lee, S.-C.; Ha, N.-R.; Jung, I.-P.; Schlesinger, S.R.; Shackelford, K.; Yoon, M.-Y.; Kim, S.-K. Inhibition of anthrax lethal factor by ssDNA aptamers. Arch. Biochem. Biophys. 2018, 646, 16–23. [Google Scholar] [CrossRef]
- Biondi, E.; Lane, J.D.; Das, D.; Dasgupta, S.; Piccirilli, J.A.; Hoshika, S.; Bradley, K.M.; Krantz, B.A.; Benner, S.A. Laboratory evolution of artificially expanded DNA gives redesignable aptamers that target the toxic form of anthrax protective antigen. Nucleic. Acids. Res. 2016, 44, 9565–9577. [Google Scholar] [CrossRef] [Green Version]
- Fischer, C.; Hünniger, T.; Jarck, J.H.; Frohnmeyer, E.; Kallinich, C.; Haase, I.; Hahn, U.; Fischer, M. Food Sensing: Aptamer-Based Trapping of Bacillus cereus Spores with Specific Detection via Real Time PCR in Milk. J. Agric. Food Chem. 2015, 63, 8050–8057. [Google Scholar] [CrossRef]
- Yan, W.; Gu, L.; Ren, W.; Ma, X.; Qin, M.; Lyu, M.; Wang, S. Recognition of Helicobacter pylori by protein-targeting aptamers. Helicobacter 2019, 24, 1–10. [Google Scholar] [CrossRef]
- Stoltenburg, R.; Schubert, T.; Strehlitz, B. In vitro selection and interaction studies of a DNA aptamer targeting Protein, A. PLoS ONE 2015, 10, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Gan, L.; Jiang, L.; Zhang, X.; Yang, X.; Chen, M.; Lan, X. Neutralization of staphylococcal enterotoxin B by an aptamer antagonist. Antimicrob. Agents. Chemother. 2015, 59, 2072–2077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramlal, S.; Mondal, B.; Lavu, P.S.; Bhavanashri, N.; Kingston, J. Capture and detection of Staphylococcus aureus with dual labeled aptamers to cell surface components. Int. J. Food Microbiol. 2018, 265, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Stoltenburg, R.; Krafčiková, P.; Víglaský, V.; Strehlitz, B. G-quadruplex aptamer targeting Protein A and its capability to detect Staphylococcus aureus demonstrated by ELONA. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Stoltenburg, R.; Strehlitz, B. Refining the Results of a Classical SELEX Experiment by Expanding the Sequence Data Set of an Aptamer Pool Selected for Protein, A. Int. J. Mol. Sci. 2018, 19, 642. [Google Scholar] [CrossRef] [Green Version]
- Sedighian, H.; Halabian, R.; Amani, J.; Heiat, M.; Amin, M.; Fooladi, A.A.I. Staggered Target SELEX, a novel approach to isolate non-cross-reactive aptamer for detection of SEA by apta-qPCR. J. Biotechnol. 2018, 286, 45–55. [Google Scholar] [CrossRef]
- Sedighian, H.; Halabian, R.; Amani, J.; Heiat, M.; Taheri, R.A.; Imani Fooladi, A.A. Manufacturing of a novel double-function ssDNA aptamer for sensitive diagnosis and efficient neutralization of SEA. Anal. Biochem. 2018, 548, 69–77. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, X.; Duan, N.; Wu, S.; Wang, Z.; Wei, X.; Wang, Y. Selection and characterization of DNA aptamers against Staphylococcus aureus enterotoxin C1. Food Chem. 2015, 166, 623–629. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, Y.; Yao, Q.; He, F. Selection of a new Mycobacterium tuberculosis H37Rv aptamer and its application in the construction of a SWCNT/aptamer/Au-IDE MSPQC H37Rv sensor. Biosens. Bioelectron. 2017, 98, 261–266. [Google Scholar] [CrossRef]
- Aimaiti, R.; Qin, L.; Cao, T.; Yang, H.; Wang, J.; Lu, J.; Huang, X.; Hu, Z. Identification and application of ssDNA aptamers against H₃₇Rv in the detection of Mycobacterium tuberculosis. Appl. Microbiol. Biotechnol. 2015, 99, 9073–9083. [Google Scholar] [CrossRef]
- Ansari, N.; Ghazvini, K.; Ramezani, M.; Shahdordizadeh, M.; Yazdian-Robati, R.; Abnous, K.; Taghdisi, S.M. Selection of DNA aptamers against Mycobacterium tuberculosis Ag85A, and its application in a graphene oxide-based fluorometric assay. Microchim. Acta 2017, 185, 21. [Google Scholar] [CrossRef]
- Yu, X.; Chen, F.; Wang, R.; Li, Y. Whole-bacterium SELEX of DNA aptamers for rapid detection of E. coli O157:H7 using a QCM sensor. J. Biotechnol. 2018, 266, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Shorie, M.; Sharma, M.; Ganguli, A.K.; Sabherwal, P. Bridged Rebar Graphene functionalized aptasensor for pathogenic E. coli O78:K80:H11 detection. Biosens. Bioelectron. 2017, 98, 486–493. [Google Scholar] [CrossRef]
- Bayraç, A.T.; Donmez, S.I. Selection of DNA aptamers to Streptococcus pneumonia and fabrication of graphene oxide based fluorescent assay. Anal. Biochem. 2018, 556, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Song, M.S.; Sekhon, S.S.; Shin, W.R.; Kim, H.C.; Min, J.; Ahn, J.Y.; Kim, Y.H. Detecting and discriminating shigella sonnei using an aptamer-based fluorescent biosensor platform. Molecules 2017, 22, 825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayrac, C.; Eyidogan, F.; Oktem, H.A. DNA aptamer-based colorimetric detection platform for Salmonella Enteritidis. Biosens.Bioelectron. 2017, 98, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Mirzakhani, K.; Gargari, S.L.M.; Rasooli, I.; Rasoulinejad, S. Development of a DNA aptamer for screening Neisseria meningitidis serogroup b by cell SELEX. Iran. Biomed. J. 2018, 22, 193–201. [Google Scholar] [CrossRef]
- Chinnappan, R.; AlAmer, S.; Eissa, S.; Rahamn, A.A.; Abu Salah, K.M.; Zourob, M. Fluorometric graphene oxide-based detection of Salmonella enteritis using a truncated DNA aptamer. Microchim. Acta 2018, 185, 1–9. [Google Scholar] [CrossRef]
- Shin, W.R.; Sekhon, S.S.; Kim, S.G.; Rhee, S.J.; Yang, G.N.; Won, K.; Rhee, S.K.; Ryu, H.; Kim, K.; Min, J.; et al. Aptamer-based pathogen monitoring for salmonella enterica ser. Typhimurium. J. Biomed. Nanotechnol. 2018, 14, 1992–2002. [Google Scholar] [CrossRef]
- Rasoulinejad, S.; Gargari, S.L.M. Aptamer-nanobody based ELASA for specific detection of Acinetobacter baumannii isolates. J. Biotechnol. 2016, 231, 46–54. [Google Scholar] [CrossRef]
- Liu, M.; Yin, Q.; Brennan, J.D.; Li, Y. Selection and characterization of DNA aptamers for detection of glutamate dehydrogenase from Clostridium difficile. Biochimie 2018, 145, 151–157. [Google Scholar] [CrossRef]
- Frohnmeyer, E.; Frisch, F.; Falke, S.; Betzel, C.; Fischer, M. Highly affine and selective aptamers against cholera toxin as capture elements in magnetic bead-based sandwich ELAA. J. Biotechnol. 2018, 269, 35–42. [Google Scholar] [CrossRef]
- Lavu, P.S.; Mondal, B.; Ramlal, S.; Murali, H.S.; Batra, H.V. Selection and Characterization of Aptamers Using a Modified Whole Cell Bacterium SELEX for the Detection of Salmonella enterica Serovar Typhimurium. Acs. Comb. Sci. 2016, 18, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Ahn, J.Y.; Lee, K.A.; Um, H.J.; Sekhon, S.S.; Sun Park, T.; Min, J.; Kim, Y.H. Analytical bioconjugates, aptamers, enable specific quantitative detection of Listeria monocytogenes. Biosens. Bioelectron. 2015, 68, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.L.; Yancey, K.; Battistella, L.; Williams, R.M.; Hickey, K.M.; Bostick, C.D.; Gannett, P.M.; Sooter, L.J. Selection of Single-Stranded DNA Molecular Recognition Elements against Exotoxin A Using a Novel Decoy-SELEX Method and Sensitive Detection of Exotoxin A in Human Serum. Biomed. Res. Int. 2015, 2015, 417641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, W.R.; Sekhon, S.S.; Rhee, S.K.; Ko, J.H.; Ahn, J.Y.; Min, J.; Kim, Y.H. Aptamer-Based Paper Strip Sensor for Detecting Vibrio fischeri. Acs. Comb. Sci. 2018, 20, 261–268. [Google Scholar] [CrossRef]
- Shin, H.S.; Gedi, V.; Kim, J.K.; Lee, D.k. Detection of Gram-negative bacterial outer membrane vesicles using DNA aptamers. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Hedayati Ch, M.; Amani, J.; Sedighian, H.; Amin, M.; Salimian, J.; Halabian, R.; Imani Fooladi, A.A. Isolation of a new ssDNA aptamer against staphylococcal enterotoxin B based on CNBr-activated sepharose-4B affinity chromatography. J. Mol. Recognit. 2016, 29, 436–445. [Google Scholar] [CrossRef]
- Fan, Y.; Cui, M.; Liu, Y.; Jin, M.; Zhao, H. Selection and characterization of DNA aptamers for constructing colorimetric biosensor for detection of PBP2a. Spectrochim. Acta A 2019. [Google Scholar] [CrossRef]
- Das, R.; Dhiman, A.; Mishra, S.K.; Haldar, S.; Sharma, N.; Bansal, A.; Ahmad, Y.; Kumar, A.; Tyagi, J.S.; Sharma, T.K. Structural switching electrochemical DNA aptasensor for the rapid diagnosis of tuberculous meningitis. Int. J. Nanomed. 2019, 14, 2103–2113. [Google Scholar] [CrossRef] [Green Version]
- Bai, L.; Chen, Y.; Bai, Y.; Chen, Y.; Zhou, J.; Huang, A. Fullerene-doped polyaniline as new redox nanoprobe and catalyst in electrochemical aptasensor for ultrasensitive detection of Mycobacterium tuberculosis MPT64 antigen in human serum. Biomaterials 2017, 133, 11–19. [Google Scholar] [CrossRef]
- Sypabekova, M.; Jolly, P.; Estrela, P.; Kanayeva, D. Electrochemical aptasensor using optimized surface chemistry for the detection of Mycobacterium tuberculosis secreted protein MPT64 in human serum. Biosens. Bioelectron. 2019, 123, 141–151. [Google Scholar] [CrossRef]
- Lavania, S.; Das, R.; Dhiman, A.; Myneedu, V.P.; Verma, A.; Singh, N.; Sharma, T.K.; Tyagi, J.S. Aptamer-Based TB Antigen Tests for the Rapid Diagnosis of Pulmonary Tuberculosis: Potential Utility in Screening for Tuberculosis. Acs. Infect. Dis. 2018, 4, 1718–1726. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, Y.; Duan, S.; Su, L.; Zhang, J.; He, F. Mycobacterium tuberculosis strain H37Rv Electrochemical Sensor Mediated by Aptamer and AuNPs-DNA. Acs. Sens. 2019, 4, 849–855. [Google Scholar] [CrossRef]
- Borsa, B.A.; Tuna, B.G.; Hernandez, F.J.; Hernandez, L.I.; Bayramoglu, G.; Arica, M.Y.; Ozalp, V.C. Staphylococcus aureus detection in blood samples by silica nanoparticle-oligonucleotides conjugates. Biosens. Bioelectron. 2016, 86, 27–32. [Google Scholar] [CrossRef]
- Cheng, D.; Yu, M.; Fu, F.; Han, W.; Li, G.; Xie, J.; Song, Y.; Swihart, M.T.; Song, E. Dual Recognition Strategy for Specific and Sensitive Detection of Bacteria Using Aptamer-Coated Magnetic Beads and Antibiotic-Capped Gold Nanoclusters. Anal. Chem. 2016, 88, 820–825. [Google Scholar] [CrossRef]
- Gao, W.; Li, B.; Yao, R.; Li, Z.; Wang, X.; Dong, X.; Qu, H.; Li, Q.; Li, N.; Chi, H.; et al. Intuitive Label-Free SERS Detection of Bacteria Using Aptamer-Based in Situ Silver Nanoparticles Synthesis. Anal. Chem. 2017, 89, 9836–9842. [Google Scholar] [CrossRef]
- Pang, Y.; Wan, N.; Shi, L.; Wang, C.; Sun, Z.; Xiao, R.; Wang, S. Dual-recognition surface-enhanced Raman scattering(SERS)biosensor for pathogenic bacteria detection by using vancomycin-SERS tags and aptamer-Fe3O4@Au. Anal. Chim. Acta 2019, 1077, 288–296. [Google Scholar] [CrossRef]
- Lian, Y.; He, F.; Wang, H.; Tong, F. A new aptamer/graphene interdigitated gold electrode piezoelectric sensor for rapid and specific detection of Staphylococcus aureus. Biosens. Bioelectron. 2015, 65, 334–339. [Google Scholar] [CrossRef]
- Sundararaj, N.; Kalagatur, N.K.; Mudili, V.; Krishna, K.; Antonysamy, M. Isolation and identification of enterotoxigenic Staphylococcus aureus isolates from Indian food samples: Evaluation of in-house developed aptamer linked sandwich ELISA (ALISA) method. J. Food Sci. Technol. 2019, 56, 1016–1026. [Google Scholar] [CrossRef]
- Ranjbar, S.; Shahrokhian, S. Designa nd fabrication of an electrochemical aptasensor using Au nanoparticles/carbon nanoparticles/cellulose nanofibers nanocomposite for rapid and sensitive detection of Staphyloccus aureus. Bioelectrochemistry 2018, 123, 70–76. [Google Scholar] [CrossRef]
- Wang, S.; Deng, W.; Yang, L.; Tan, Y.; Xie, Q.; Yao, S. Copper-Based Metal-Organic Framework Nanoparticles with Peroxidase-Like Activity for Sensitive Colorimetric Detection of Staphylococcus aureus. ACS Appl. Mater. Interfaces 2017, 9, 24440–24445. [Google Scholar] [CrossRef]
- Li, J.; Jiang, H.; Rao, X.; Liu, Z.; Zhu, H.; Xu, Y. Point-of-Care Testing of Pathogenic Bacteria at the Single-Colony Level via Gas Pressure Readout Using Aptamer-Coated Magnetic CuFe 2 O 4 and Vancomycin-Capped Platinum Nanoparticles. Anal. Chem. 2019, 91, 1494–1500. [Google Scholar] [CrossRef]
- Yu, T.; Xu, H.; Zhao, Y.; Han, Y.; Zhang, Y.; Zhang, J.; Xu, C.; Wang, W.; Guo, Q.; Ge, J. Aptamer based high throughput colorimetric biosensor for detection of staphylococcus aureus. Sci. Rep. 2020, 10, 1–6. [Google Scholar] [CrossRef]
- Xu, J.; Guo, J.; Maina, S.W.; Yang, Y.; Hu, Y.; Li, X.; Qiu, J.; Xin, Z. An aptasensor for staphylococcus aureus based on nicking enzyme amplification reaction and rolling circle amplification. Anal. Biochem. 2018, 549, 136–142. [Google Scholar] [CrossRef]
- Wang, J.; Wu, X.; Wang, C.; Shao, N.; Dong, P.; Xiao, R.; Wang, S. Magnetically Assisted Surface-Enhanced Raman Spectroscopy for the Detection of Staphylococcus aureus Based on Aptamer Recognition. ACS Appl. Mater. Interfaces 2015, 7, 20919–20929. [Google Scholar] [CrossRef]
- Cai, R.; Yin, F.; Chen, H.; Tian, Y.; Zhou, N. A fluorescent aptasensor for Staphylococcus aureus based on strand displacement amplification and self-assembled DNA hexagonal structure. Microchim. Acta 2020, 187, 604–620. [Google Scholar] [CrossRef]
- Qiao, J.; Meng, X.; Sun, Y.; Li, Q.; Zhao, R.; Zhang, Y.; Wang, J.; Yi, Z. Aptamer-based fluorometric assay for direct identification of methicillin-resistant Staphylococcus aureus from clinical samples. J. Microbiol. Methods 2018, 153, 92–98. [Google Scholar] [CrossRef]
- Oscoy, I.; Yusuflbeyoglu, S.; Yilmaz, V.; McLamore, E.S.; Ildiz, N.; Ulgen, A. DNA aptamer functionalized gold nanostructores for molecular recognition and photothermal inactivation of methicillin-Resistant Satphylococcus aureus. Colloids Surf. B 2017, 159, 16–22. [Google Scholar]
- Xu, Y.; Wang, H.; Luan, C.; Liu, Y.; Chen, B.; Zhao, Y. Aptamer-based hydrogel barcodes for the capture and detection of multiple types of pathogenic bacteria. Biosens. Bioelectron. 2018, 100, 404–410. [Google Scholar] [CrossRef]
- Jin, B.; Wang, S.; Lin, M.; Jin, Y.; Zhang, S.; Cui, X.; Gong, Y.; Li, A.; Xu, F.; Lu, T.J. Upconversion nanoparticles based FRET aptasensor for rapid and ultrasensitive bacteria detection. Biosens. Bioelectron. 2017, 90, 525–533. [Google Scholar] [CrossRef]
- Jo, N.; Kim, H.; Lee, S.-M.; Oh, J.; Park, I.H.; Lim, K.J.; Shin, J.-s.; Yoo, K.H. Aptamer-functionalized capacitance sensors for real-time monitoring of bacterial growna dn antibiotics susceptibility. Biosensors 2018, 102, 164–170. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Yang, Q.; Jiang, X.; Li, Y.; Zhao, J.; Qu, K. Conductometric sensor for viable Escherichia coli and Staphylococcus aureus based on magnetic analyte separation via aptamer. Microchim. Acta 2020, 187, 43. [Google Scholar] [CrossRef]
- Wu, W.; Zhao, S.; Mao, Y.; Fang, Z.; Lu, X.; Zeng, L. A sensitive lateral flow biosensor for Escherichia coli O157: H7 detection based on aptamer mediated strand displacement amplification. Anal. Chim. Acta 2015, 861, 62–68. [Google Scholar] [CrossRef]
- Khang, J.; Kim, D.; Chung, K.W.; Lee, J.H. Chemiluminescent aptasensor capable of rapidly quantifying Escherichia coli O157:H7. Talanta 2016, 147, 177–183. [Google Scholar] [CrossRef]
- Shahrokhian, S.; Ranjbar, S. Aptamer immobilization on amino-functionalized metal-organic frameworks: An ultrasensitive platform for the electrochemical diagnostic of Escherichia coli O157:H7. Analyst 2018, 143, 3191–3201. [Google Scholar] [CrossRef]
- Bu, S.; Wang, K.; Li, Z.; Wang, C.; Hao, Z.; Liu, W.; Wan, J. An electrochemical biosensor based on methylene blue-loaded nanocomposites as signal-amplifying tags to detect pathogenic bacteria. Analyst 2020. [Google Scholar] [CrossRef]
- Dong, X.; Shi, Z.; Xu, C.; Yang, C.; Chen, F.; Lei, M. CdS quantum dots/Au nanoparticles/ZnO nanowire array for self-powered photoelectrochemical detection of Escherichia coli O157:H7. Biosens. Bioelectron. 2020, 149, 111843. [Google Scholar] [CrossRef]
- Ling, M.; Peng, Z.; Cheng, L.; Deng, L. Rapid fluorescent detection of enterotoxigenic Escherichia coli (ETEC) K88 based on graphene oxide-dependent nanoquencher and Klenow fragment-triggered target cyclic amplification. Appl. Spectrosc. 2015, 69, 1175–1181. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, Q.; Xu, T.; Wang, F.; Huang, F.; Peng, Y.; Deng, L. G-quadruplex-based assay combined with aptamer and gold nanoparticles for Escherichia coli K88 determination. Microchim. Acta 2020, 187, 604–620. [Google Scholar] [CrossRef]
- Hao, N.; Zhang, X.; Zhou, Z.; Hua, R.; Zhang, Y.; Liu, Q.; Qian, J.; Li, H.; Wang, K. AgBr nanoparticles/3D nitrogen-doped graphene hydrogel for fabricating all-solid-state luminol-electrochemiluminescence Escherichia coli aptasensors. Biosens. Bioelectron. 2017, 97, 377–383. [Google Scholar] [CrossRef]
- Hua, R.; Hao, N.; Lu, J.; Qian, J.; Liu, Q.; Li, H.; Wang, K. A sensitive Potentiometric resolved ratiometric Photoelectrochemical aptasensor for Escherichia coli detection fabricated with non-metallic nanomaterials. Biosens. Bioelectron. 2018, 106, 57–63. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Y.; Liu, S.; Yu, J.; Wang, H.; Wang, Y.; Huang, J. Label-free and highly sensitive electrochemical detection of E. coli based on rolling circle amplifications coupled peroxidase-mimicking DNAzyme amplification. Biosens. Bioelectron. 2016, 75, 315–319. [Google Scholar] [CrossRef]
- Wu, G.; Dai, Z.; Tang, X.; Lin, Z.; Lo, P.K.; Meyyappan, M.; Lai, K.W.C. Graphene Field-Effect Transistors for the Sensitive and Selective Detection of Escherichia coli Using Pyrene-Tagged DNA Aptamer. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef]
- Abdelrasoul, G.N.; Anwar, A.; MacKay, S.; Tamura, M.; Shah, M.A.; Khasa, D.P.; Montgomery, R.R.; Ko, A.I.; Chen, J. DNA aptamer-based non-faradaic impedance biosensor for detecting E. coli. Anal. Chim. Acta 2020, 1107, 135–144. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, Y.; Zhang, Z.; Wei, J.; Xie, S.; Li, X. Fibrous testing papers for fluorescence trace sensing and photodynamic destruction of antibiotic-resistant bacteria. J. Mater. Chem. B 2020, 8, 2709–2718. [Google Scholar] [CrossRef]
- Bruno, J.G.; Sivils, J.C.; Phillips, T. Aptamer-magnetic bead quantum dot sandwich assays for foodborne pathogen detection: Pros, cons, and lessons learned. J. Aoac. Int. 2017, 100, 895–899. [Google Scholar] [CrossRef]
- Yoo, S.M.; Kim, D.K.; Lee, S.Y. Aptamer-functionalized localized surface plasmon resonance sensor for the multiplexed detection of different bacterial species. Talanta 2015, 132, 112–117. [Google Scholar] [CrossRef]
- Das, R.; Dhiman, A.; Kapil, A.; Bansal, V.; Sharma, T.K. Aptamer-mediated colorimetric and electrochemical detection of Pseudomonas aeruginosa utilizing peroxidase-mimic activity of gold NanoZyme. Anal. Bioanal. Chem. 2019, 411, 1229–1238. [Google Scholar] [CrossRef]
- Zhong, Z.; Gao, X.; Gao, R.; Jia, L. Selective capture and sensitive fluorometric determination of Pseudomonas aeruginosa by using aptamer modified magnetic nanoparticles. Mikrochim. Acta 2018, 185, 377. [Google Scholar] [CrossRef]
- Wu, Z.; He, D.; Cui, B.; Jin, Z. A bimodal (SERS and colorimetric) aptasensor for the detection of Pseudomonas aeruginosa. Microchim. Acta 2018, 185, 1–7. [Google Scholar] [CrossRef]
- Hu, J.; Fu, K.; Bohn, P.W. Whole-Cell Pseudomonas aeruginosa Localized Surface Plasmon Resonance Aptasensor. Anal. Chem. 2018, 90, 2326–2332. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Xie, G.; Gou, D.; Luo, P.; Yao, Y.; Chen, H. A novel enzyme-free electrochemical biosensor for rapid detection of Pseudomonas aeruginosa based on high catalytic Cu-ZrMOF and conductive Super, P. Biosens. Bioelectron. 2019, 142. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, J.; Estévez, M.C.; Fernández-Gavela, A.; González-López, J.J.; González-Guerrero, A.B.; Lechuga, L.M. Label-free detection of nosocomial bacteria using a nanophotonic interferometric biosensor. Analyst 2020, 145, 497–506. [Google Scholar] [CrossRef]
- Wu, J.H.; Wang, C.H.; Ma, Y.D.; Lee, G.B. A nitrocellulose membrane-based integrated microfluidic system for bacterial detection utilizing magnetic-composite membrane microdevices and bacteria-specific aptamers. Lab. Chip. 2018, 18, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Su, C.H.; Tsai, M.H.; Lin, C.Y.; Ma, Y.D.; Wang, C.H.; Chung, Y.D.; Lee, G.B. Dual aptamer assay for detection of Acinetobacter baumannii on an electromagnetically-driven microfluidic platform. Biosens. Bioelectron. 2020, 159, 112148. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Guo, Y.; Fan, J.; Yang, Y.; Zuo, C.; Bai, S.; Sheng, S.; Li, J.; Xie, G. A fluorometric assay for rapid enrichment and determination of bacteria by using zirconium-metal organic frameworks as both capture surface and signal amplification tag. Microchim. Acta 2020, 187, 604–620. [Google Scholar] [CrossRef] [PubMed]
- Hills, K.D.; Oliveira, D.A.; Cavallaro, N.D.; Gomes, C.L.; McLamore, E.S. Actuation of chitosan-aptamer nanobrush borders for pathogen sensing. Analyst 2018, 143, 1650–1661. [Google Scholar] [CrossRef] [Green Version]
- Pei, Q.; Song, X.; Liu, S.; Wang, J.; Leng, X.; Cui, X.; Yu, J.; Wang, Y.; Huang, J. A facile signal-on electrochemical DNA sensing platform for ultrasensitive detection of pathogenic bacteria based on Exo III-assisted autonomous multiple-cycle amplification. Analyst 2019, 144, 3023–3029. [Google Scholar] [CrossRef]
- Park, K.S.; Charles, R.C.; Ryan, E.T.; Weissleder, R.; Lee, H. Fluorescence polarization-based nucleic acid testing for rapid and cost-effective diagnosis of infectious disease. Chemistry 2015, 21. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Zhou, W.; Sanjay, S.T.; Zhang, J.; Jin, Q.; Xu, F.; Dominguez, D.C.; Li, X. Multiplexed Instrument-Free Bar-Chart SpinChip Integrated with Nanoparticle-Mediated Magnetic Aptasensors for Visual Quantitative Detection of Multiple Pathogens. Anal. Chem. 2018, 90, 9888–9896. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, X.; Liu, Y.; Duan, N.; Wu, S.; Wang, Z.; Xu, B. Gold nanoparticles enhanced SERS aptasensor for the simultaneous detection of Salmonella typhimurium and Staphylococcus aureus. Biosens. Bioelectron. 2015, 74, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhou, X.; Shan, Y.; Yue, H.; Huang, R.; Hu, J.; Xing, D. Sensitive detection of a bacterial pathogen using allosteric probe-initiated catalysis and CRISPR-Cas13a amplification reaction. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruno, J.G.; Phillips, T.; Montez, T.; Garcia, A.; Sivils, J.C.; Mayo, M.W.; Greis, A. Development of a fluorescent enzyme-linked DNA aptamer-magnetic bead sandwich assay and portable fluorometer for sensitive and rapid listeria Detection. J. Fluoresc. 2015, 25, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.x.; Lv, J.j.; Chen, L.; Li, S.b.; Mou, X.j.; Xu, Y. A fluorescent probe composed of quantum dot labeled aptamer and graphene oxide for the determination of the lipopolysaccharide endotoxin. Microchim. Acta 2019, 186, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Oueslati, R.; Cheng, C.; Zhao, L.; Chen, J.; Almeida, R.; Wu, J. Rapid, highly sensitive detection of Gram-negative bacteria with lipopolysaccharide absed disposable aptasensor. Biosens. Bioelectron. 2018, 112, 48–53. [Google Scholar] [CrossRef] [Green Version]
- Niu, J.; Hu, X.; Ouyang, W.; Chen, Y.; Liu, S.; Han, J.; Liu, L. Femtomolar Detection of Lipopolysaccharide in Injectables and Serum Samples Using Aptamer-Coupled Reduced Graphene Oxide in a Continuous Injection-Electrostacking Biochip. Anal. Chem. 2019, 91, 2360–2367. [Google Scholar] [CrossRef]
- Zhu, L.; Li, S.; Shao, X.; Feng, Y.; Xie, P.; Luo, Y.; Huang, K.; Xu, W. Colorimetric detection and typing of E. coli lipopolysaccharides based on a dual aptamer-functionalized gold nanoparticle probe. Microchim. Acta 2019, 186, 6–11. [Google Scholar] [CrossRef]
- Posha, B.; Sandhyarani, N. Highly sensitive endotoxin detection using a gold nanoparticle loaded layered molybdenum disulfide-polyacrylic acid nanocomposite. Analyst 2020, 145, 3939–3947. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Shayeh, J.S.; Omidi, M.; Yazdian, F.; Alebouyeh, M. A glassy carbon electrode modified with reduced graphene oxide and gold nanoparticles for electrochemical aptasensing of lipopolysaccharides from Escherichia coli bacteria. Microchim. Acta 2019, 186, 787. [Google Scholar] [CrossRef]
- Xie, P.; Zhu, L.; Shao, X.; Huang, K.; Tian, J.; Xu, W. Highly sensitive detection of lipopolysaccharides using an aptasensor based on hybridization chain reaction. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, H.; Duan, N.; Gu, H.; Wang, H.; Wang, Z. Fluorometric determination of lipopolysaccharides via changes of the graphene oxide-enhanced fluorescence polarization caused by truncated aptamers. Microchim. Acta 2019, 186, 3–10. [Google Scholar] [CrossRef]
- Mazzaracchio, V.; Neagu, D.; Porchetta, A.; Marcoccio, E.; Pomponi, A.; Faggioni, G.; D’Amore, N.; Notargiacomo, A.; Pea, M.L.i.; Moscone, D.; et al. A label-free impedimetric aptasensor for the detection of Bacillus anthracis spore simulant. Biosens. Bioelectron. 2019, 126, 640–646. [Google Scholar] [CrossRef]
- Wu, Z.; He, D.; Cui, B. A fluorometric assay for staphylococcal enterotoxin B by making use of platinum coated gold nanorods and of upconversion nanoparticles. Microchim. Acta 2018, 185, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Mousavi Nodoushan, S.; Nasirizadeh, N.; Amani, J.; Halabian, R.; Imani Fooladi, A.A. An electrochemical aptasensor for staphylococcal enterotoxin B detection based on reduced graphene oxide and gold nano-urchins. Biosens. Bioelectron. 2019, 127, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Mondal, B.; Ramlal, S.; Lavu, P.S.; Bhavanashri, N.; Kingston, J. Highly sensitive colorimetric biosensor for staphylococcal enterotoxin B by a label-free aptamer and gold nanoparticles. Front. Microbiol. 2018, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, H.; Chen, X.; Wang, X.; Duan, N.; Wu, S.; Xu, B.; Wang, Z. A multicolor time-resolved fluorescence aptasensor for the simultaneous detection of multiplex Staphylococcus aureus enterotoxins in the milk. Biosens. Bioelectron. 2015, 74, 170–176. [Google Scholar] [CrossRef]
- Zhang, L.; Qi, Z.; Zou, Y.; Zhang, J.; Xia, W.; Zhang, R.; He, Z.; Cai, X.; Lin, Y.; Duan, S.-Z.; et al. Engineering DNA − Nanozyme Interfaces for Rapid Detection of Dental Bacteria. ACS Appl. Mater. Interfaces 2019, 11, 30640–30647. [Google Scholar] [CrossRef]
- Zhao, G.; Ding, J.; Yu, H.; Yin, T.; Qin, W. Potentiometric aptasensing of Vibrio alginolyticus based on DNA nanostructure-modified magnetic beads. Sensors 2016, 16, 2052. [Google Scholar] [CrossRef]
- Sun, Y.; Duan, N.; Ma, P.; Liang, Y.; Zhu, X.; Wang, Z. Colorimetric Aptasensor Based on Truncated Aptamer and Trivalent DNAzyme for Vibrio parahemolyticus Determination. J. Agric. Food Chem. 2019, 67, 2313–2320. [Google Scholar] [CrossRef]
- Oroval, M.; Coronado-Puchau, M.; Langer, J.; Sanz-Ortiz, M.N.; Ribes, Á.; Aznar, E.; Coll, C.; Marcos, M.D.; Sancenón, F.; Liz-Marzán, L.M.; et al. Surface Enhanced Raman Scattering and Gated Materials for Sensing Applications: The Ultrasensitive Detection of Mycoplasma and Cocaine. Chem. A. Eur. J. 2016, 22, 13488–13495. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.; Stoltenburg, R.; Strehlitz, B.; Frense, D.; Beckmann, D. Development of An Impedimetric Aptasensor for the Detection of Staphylococcus aureus. Int. J. Mol. Sci. 2017, 18, 2484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urmann, K.; Reich, P.; Walter, J.G.; Beckmann, D.; Segal, E.; Scheper, T. Rapid and label-free detection of protein a by aptamer-tethered porous silicon nanostructures. J. Biotechnol. 2017, 257, 171–177. [Google Scholar] [CrossRef]
- Oliveira, D.; Borges, A.; Simões, M. Staphylococcus aureus Toxins and Their Molecular Activity in Infectious Diseases. Toxins 2018, 10, 252. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.S.; de Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers 2018, 4, 18033. [Google Scholar] [CrossRef]
- Ehrt, S.; Schnappinger, D.; Rhee, K.Y. Metabolic principles of persistence and pathogenicity in Mycobacterium tuberculosis. Nat. Rev. Microbiol. 2018, 16, 496–507. [Google Scholar] [CrossRef]
- Hartzell, J.D.; Kim, A.S.; Kortepeter, M.G.; Moran, K.A. Acinetobacter pneumonia: A review. Med. Gen. Med. 2007, 9, 4. [Google Scholar]
- Weiser, J.N.; Ferreira, D.M.; Paton, J.C. Streptococcus pneumoniae: Transmission, colonization and invasion. Nat. Rev. Microbiol. 2018, 16, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Moradali, M.F.; Ghods, S.; Rehm, B.H.A. Pseudomonas aeruginosa Lifestyle: A Paradigm for Adaptation, Survival, and Persistence. Front. Cell Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef] [Green Version]
- Ehling-Schulz, M.; Lereclus, D.; Koehler, T.M. The Bacillus cereus Group: Bacillus Species with Pathogenic Potential. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Gao, R.; Li, D.; Lin, Y.; Lin, J.; Xia, X.; Wang, H.; Bi, L.; Zhu, J.; Hassan, B.; Wang, S.; et al. Structural and Functional Characterization of the FadR Regulatory Protein from Vibrio alginolyticus. Front. Cell Infect. Microbiol. 2017, 7, 513. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Liu, X.; Zeng, Z.; Chen, Z.; Liu, Y.; Zu, Y. Aptamer Cocktail to Detect Multiple Species of Mycoplasma in Cell Culture. Int. J. Mol. Sci. 2020, 21, 3784. [Google Scholar] [CrossRef] [PubMed]







| Target | SELEX Method | Equilibrium Dissociation Constant | Class of Molecule | Reference |
|---|---|---|---|---|
| Vibrio alginolyticus | Whole cell | 14.31 ± 4.26 nM (VA2) and 90 ± 13.51 nM (VA8) | Bacterial cell | [21] |
| Vibrio parahemolyticus | Whole cell coupled with graphene oxide and isothermal amplification | 10.3 ± 2.5 nM | Bacterial cell | [22] |
| Vibrio Vulnificus | Whole cell | 26.8 ± 5.3 nM | Bacterial cell | [23] |
| Escherichia coli | Whole cell | 107.6 ± 67.8 pM | Bacterial cell | [24] |
| Different stages of E. coli (Escherichia coli) O157:H7 | Whole cell | 9.04 ± 2.80 nM | Bacterial cell | [25] |
| Escherichia coli | Whole cell | Four aptamers: range from 11.97 to 161 nM | Bacterial cell | [26] |
| E. coli DH5 alpha | Whole cell | 27.4 ± 18.7 nM | Bacterial cell | [27] |
| Streptococcus pyogenes (M type) | Flow cytometry assisted | Low nanomolar range | Bacterial cell | [28] |
| M-type 11 Streptococcus pyogenes | Whole cell | 7 ± 1 nM | Bacterial cell | [28] |
| Group A Streptococcus Serotype M3 | Whole cell | 7.47 ± 1.72 pM | Bacterial cell | [29] |
| Streptococcus mutans | Subtractive (Whole cell) SELEX | 69.45 ± 38.53 nM | Bacterial cell | [30] |
| Sepsis Bacterium (A. baumanii (Acinetobacter baumanii), L. monocytogenes (Listeria monocytogenes), E. coli, S. aureus (Staphylococcus aureus), K. pneumonia (Klebsiella pneumonia)) | Whole cell | To each bacteria in order, Antibac 1 (268.5 ± 54.34, 51.74 ± 11.75, 31.82 ± 4.38, 170.10 ± 32.13, 256.10 ± 47.89) nM; Antibac 2 (71.92 ± 9.74, 54.19 ± 12.09, 62.43 ± 11.97, 194.90 ± 38.55, 195.90 ± 42.91) nM | Bacterial cell | [31] |
| MPT 64 (Mycobacterium tuberculosis secretory protein) | Nitrocellulose membrane | 8.92 nM | Protein | [32] |
| Mycobacterium Tuberculosis H37RA | Whole cell | 5.09 ± 1.43 nM | Bacterial cell | [33] |
| Mannose-Capped Lipoarabinomannan of Bacillus Calmette–Guérin | 96-well plate | 8.59 ± 1.23 nM | Lipoglycan | [34] |
| Mannose-Capped Lipoarabinomannan of Mycobacterium tuberculosis | 96-well plate | 668 ± 159 nM | Lipoglycan | [35] |
| Pseudomonas aeruginosa | Whole cell | Low nanomolar range | Bacterial cell | [36] |
| Salmonella Typhimurium | Magnetic assisted Cell SELEX | 6.33 ± 0.58 nM | Bacterial cell | [37] |
| Escherichia coli, Enterobacter aerogenes, Klebsiella pneumoniae, Citrobacter freundii, Bacillus subtilis, and Staphylococcus epidermidis | Sequential toggle cell-SELEX | 9.22 nM to 38.5 nM | Bacterial cell | [38] |
| Lethal factor (Bacillus anthracis) | Electrophoretic mobility shift assay | 11 ± 2.7 nM | Protein | [39] |
| Protective antigen (Bacillus anthracis) | Magnetic beads | 35 nM | Protein | [40] |
| Bacillus cereus spores | Unpublished | 5.2 ± 52.4 nM | Bacterial spores | [41] |
| H. pylori (Helicobacter pylori) surface recombinant antigen | 96-well plate | 26.48 ± 5.72 nM | Protein | [42] |
| Protein A (S. aureus) | FluMag-SELEX | Low to submicromolar range | Protein | [43] |
| Staphylococcal enterotoxin B | Magnetic beads | 64 nM | Protein | [44] |
| Target | SELEX Method | Kd | Detection Method | LOD | Class of Molecule | Reference |
|---|---|---|---|---|---|---|
| Shigella sonnei | whole cell | SS−3: 39.32 ± 5.02 nM and SS4: 15.89 ± 1.77 nM | Fluorescence | 103 cells per mL | Bacterial cell | [57] |
| M. tuberculosis | whole cell | 37 ± 4 nM | Piezoelectric quartz crystal | 100 CFU/mL | Bacterial cell | [51] |
| Salmonella Enteritidis | whole cell | crn−1: 0.971 µM and crn−2: 0.309 µM | Colorimetric | 103 CFU/mL | Bacterial cell | [58] |
| Neisseria meningitidis | whole cell | K3: 28.3 ± 8.9 pM K4: 39.1 ± 8.6 pM | Fluorescence | 200 CFU/mL (infected) 100 CFU/mL (artificially infected) | Bacterial cell | [59] |
| E. coli O78:K80:H11 strain | whole cell | 14 nM | Label free impedimetric | 10 CFU/mL | Bacterial cell | [55] |
| Salmonella enteritidis | whole cell | 80 nM | Fluorescence | 25 CFU/mL | Bacteria cell | [60] |
| Salmonella enterica ser. Typhimurium | whole cell | 0.00214 ± 0.00312 µM | Fluorescence | 2 × 101 to 2 × 105 CFU/mL | Bacterial cell | [61] |
| Staphylococcal enterotoxin A (SEA) | whole cell | 8.5 ± 0.91 nM | Surface plasmon resonance | 5 ng/mL | Protein | [49] |
| Acinetobacter baumanii | whole cell | Aci49: 7.547 ± 1.353 pM Aci55: 10.70 ± 2.561 pM | Colorimetric (ELASA) | 103 CFU/mL | Bacterial cell | [62] |
| Glutamate dehydrogenase (Clostridium difficile) | Magnetic beads | anti-GDH1: 3.1 ± 1.2 nM anti-GDH3: 5.6 ± 2.4 nM anti-GDH7: 4.6 ± 1.6 nM | FRET | 1 nM | Protein | [63] |
| Streptococcus pneumonia | Whole cell | Lyd−1: 844.7 ± 123.6 nM Lyd−2: 1984.8 ± 347.5 nM Lyd−3: 661.8 ± 111.3 nM | GO based fluorescent assay | 15 CFU/mL | Protein | [56] |
| Staphylococcal enterotoxin A (SEA) | Staggered target SELEX | 7.44 + 0.6 nM | Apta-qPCR | 146.67 fM | Bacterial cell | [48] |
| E. coli O157:H7 | Whole cell | 10.30 nM | Quartz crystal microbalance | 1.46 × 103 CFU/mL | Bacterial cell | [54] |
| Cholera Toxin | Semi-automated | 23.2 - 56 nM | Sandwich enzyme linked aptamer assay | 2.1 ng/mL (binding buffer) 2.4 ng/mL (tap water) | Protein | [64] |
| M. tuberculosis H37Rv strain | Whole cell | 12.02 nM | Sandwich ELISA assay | 1 × 103 CFU/mL | Bacterial cell | [52] |
| Staphylococcus aureus | Whole cell | 34 to 128 nM | Colorimetric | 102 CFU/mL | Bacterial cell | [45] |
| Staphylococcus aureus enterotoxin C1 | Whole cell | 65.14 ± 11.64 nM | Fluorescence | 6 ng/mL | Protein | [50] |
| Salmonella enterica serovar typhimurium | Whole cell | SAL28: 195 + 46 nM SAL 11: 184 + 43 nM SAL 26: 123 + 23 nM | Fluorescence | 103 CFU/mL | Bacterial cell | [65] |
| Listeria monocytogenes | Whole cell | LMCA2: 2.01 × 10−12 M LMCA 26: 1.56 × 10−10 M | Fluorescence | 20 CFU/mL | Bacterial cell | [66] |
| Mycobacterium tuberculosis Ag85A, | Magnetic beads | 63 nM | GO based fluorescent assay | 1.5 nM | Protein | [53] |
| Pseudomonas aeruginosa exotoxin A | Magnetic beads | 4.2 to 4.5 µM | Sandwich aptamer modified ELISA assay | 100 nM | Protein | [67] |
| Vibro fischeri | Whole cell | VFCA−02: 1.28 × 10−10 M VFCA−03: 25 × 10−9 M | Colorimetric | 4 × 101 CFU/mL | Bacterial cell | [68] |
| Gram-negative bacterial outer membrane vesicles | Toggle-cell-SELEX | 20.36 to 59.70 nM | Enzyme-linked aptamer assay (ELAA) | 25 ng/mL | Outer membrane vesicles | [69] |
| Staphylococcal enterotoxin B | Affinity chromatography | 2.3 × 10−11 M | Enzyme-linked aptamer assay (ELAA) | 5 ng | Protein | [70] |
| Penicillin binding proteins | X aptamer selection kit protocol | S3,15 nM S1 30 nM | Optical Colorimetric | 20 nM | Protein | [71] |
| Target | Detection Method | Limit of Detection | Class of Molecule | Reference |
|---|---|---|---|---|
| Tuberculosis Meningitis antigens | Electrochemical Amperometric | 10 pg | Protein | [72] |
| Mycobacterium tuberculosis MPT64 antigen | Electrochemical Amperometric | 20 fg/mL | Protein | [73] |
| Mycobacterium tuberculosis MPT64 antigen | Electrochemical Impedimetric | 81 pM | Protein | [74] |
| Mycobacterium tuberculosis HspX antigen | Optical Aptamer linked immobilized sorbent assay Electrochemical Amperometric | ~13 pM | Protein | [75] |
| Mycobacterium tuberculosis strain H37Rv | Electrochemical piezoelectric quartz crystal | 100 CFU/mL | Whole cell | [76] |
| Staphylococcus aureus | Fluorescence | 682 cells | Whole cell | [77] |
| Staphylococcus aureus | Optical Colorimetric | 16 CFU/mL | Whole cell | [78] |
| Staphylococcus aureus | Surface-enhanced Raman scattering (SERS) | 1.5 CFU/mL | Whole cell | [79] |
| Staphylococcus aureus | Surface-enhanced Raman scattering (SERS) | 3 cells/mL | Whole cell | [80] |
| Staphylococcus aureus | Electrical Piezoelectric quartz crystal | 41 CFU/mL | Whole cell | [81] |
| Staphylococcus aureus | Fluorescence | 93–270 CFU/mL | Whole cell | [82] |
| Staphylococcus aureus | Electrochemical Impedimetric | 1 CFU/mL | Whole cell | [83] |
| Staphylococcus aureus | Optical Colorimetric | 20 CFU/mL | Whole cell | [84] |
| Staphylococcus aureus | Pressure Readout Using Aptamer-Coated Magnetic CuFe2O4 and Vancomycin-Capped Platinum Nanoparticles | 1 CFU/mL | Whole cell | [85] |
| Staphylococcus aureus | Colorimetric Absorbance | 81 CFU/mL | Whole cell | [86] |
| Staphylococcus aureus | Optical Chemiluminescence | 5 CFU/mL | Whole cell | [87] |
| Staphylococcus aureus | Surface-enhanced Raman scattering (SERS) | 10 cells/mL | Whole cell | [88] |
| Staphylococcus aureus | Fluorescence | 1.7 CFU/mL | Whole cell | [89] |
| methicillin-resistant Staphylococcus aureus (MRSA) | Fluorescence | 2.63 × 103 (PBS) 1.38 × 103 (spiked nasal swab) | Whole cell | [90] |
| methicillin-resistant Staphylococcus aureus (MRSA) | Optical Colorimetric | Not mentioned | Whole cell | [91] |
| E. coli and S. aureus | Optical Colorimetric | 100 CFU/mL | Whole cell | [92] |
| E. coli and S. aureus | FRET | 3 CFU/mL | Whole cell | [93] |
| E. coli and S. aureus | Electrical Capacitance sensor | 10 CFU/mL | Whole cell | [94] |
| E. coli and S. aureus | Electrical Conductometric | 2.3 × 104 CFU·mL−1 and 4.0 × 103 CFU/mL for E. coli and S. aureus | Whole cell | [95] |
| Escherichia coli O157:H7 | Optical Colorimetric Lateral flow assays | 10 CFU/mL | Whole cell | [96] |
| Escherichia coli O157:H7 | Optical Chemiluminescence | 4.5 × 103 CFU/mL | Whole cell | [97] |
| Escherichia coli O157:H7 | Electrochemical Impedimetric | 2 CFU/mL | Whole cell | [98] |
| Escherichia coli O157:H7 | Electrochemical Amperometric | 32 CFU/mL | Whole cell | [99] |
| Escherichia coli O157:H7 | Photoelectrochemical aptasensor using CdS Quantum dots/Au nanoparticles/ZnO Nanowire Array | 1.125 CFU/mL | Whole cell | [100] |
| Escherichia coli (ETEC) K88 | Fluorescence | 102 CFU/mL | Whole cell | [101] |
| Escherichia coli K88 | Optical Colorimetric | 1.35 × 102 CFU/mL | Whole cell | [102] |
| Escherichia coli | Optical Electrochemiluminescene | 0.17 CFU/mL | Whole cell | [103] |
| Escherichia coli | Electrochemical Photocurrent | 0.66 CFU/mL | Whole cell | [104] |
| Escherichia coli | Electrochemical Amperometric | 8 CFU/mL | Whole cell | [105] |
| Escherichia coli | Electrochemical Amperometric | 100 CFU/mL | bacteria | [106] |
| Escherichia coli | Non-Faradaic Impedance Biosensor | 9 CFU/mL | Whole cell | [107] |
| Antibiotic resistant E. coli | Fluorescence | 60 CFU/mL | Whole cell | [108] |
| E. coli and Salmonella | Fluorescence | 100 CFU/mL | Whole cell | [109] |
| Salmonella typhirium and Pseudomonas aeruginosa | Optical Localized Surface Plasmon Resonance | 30 CFU/mL | Whole cell | [110] |
| Pseudomonas aeruginosa | Optical Colorimetric Electrochemical Amperometric | 60 CFU/mL | Whole cell | [111] |
| Pseudomonas aeruginosa | Optical Fluorescence | 1 CFU/mL | Whole cell | [112] |
| Pseudomonas aeruginosa | Surface-enhanced Raman scattering Optical Colorimetric | 20 CFU/mL | Whole cell | [113] |
| Pseudomonas aeruginosa strain PAO1 | Optical Localized Surface Plasmon Resonance sensor | a single bacterium | Whole cell | [114] |
| Pseudomonas aeruginosa | Electrochemical Amperometric | 2 CFU/mL | bacteria | [115] |
| Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus (MRSA) | Nanophotonic Interferometric Biosensor | 49 and 29 CFU/mL was estimated for P. aeruginosa and MRSA | Whole cell | [116] |
| Acinetobacter baumannii, Escherichia coli and methicillin-resistant Staphylococcus aureus (MRSA) | Colorimetric Nitrocellulose membrane-based integrated microfluidic system | 450 CFU | Whole cell | [117] |
| Acinetobacter baumannii | Fluorescence | 100 CFU/reaction | Whole cell | [118] |
| Acinetobacter baumannii | Fluorescence | 10 CFU/mL | Whole cell | [119] |
| Campylobacter jejuni | Fluorescence | 2 CFU/mL | Whole cell | [109] |
| Listeria monocytogenes | Fluorescence | 1–10 CFU/mL | Whole cell | [109] |
| Listeria monocytogenes | Electrochemical Amperometric | 9–107 CFU/mL | Whole cell | [120] |
| Salmonella typhimurium, Bacillus subtilis, E. coli, and Listeria | Electrochemical Amperometric | 8 CFU/mL | Whole cell | [121] |
| Salmonella | Fluorescence polarization | a single bacterium | Whole cell | [122] |
| Salmonella enterica, Escherichia coli and Listeria monocytogenes | SpinChip Integrated with magnetic nanoparticles | 10 CFU/mL | Whole cell | [123] |
| Salmonella typhimurium and Saphylococcus aureus | Gold nanoparticles Surface-Enhanced Raman Scattering | 35 CFU/mL (S. aureus) 15 CFU/mL (S. typhimurium) | Whole cell | [124] |
| Salmonella Enteritidis | Allosteric Probe-Initiated Catalysis and CRISPR-Cas13a Amplification | 1 CFU/mL | Whole cell | [125] |
| Listeriolysin O protein (Listeria) | Fluorescence | 4–61 cells | Protein | [126] |
| Lipopolysaccharide | FRET | 8.7 ng/mL | Lipoglycans | [127] |
| Lipopolysaccharide of E. coli 3 strains - ATCC 25922, DH5 alpha, and field isolate | Electrical Capacitance sensor | 102 cells/mL | Whole cell | [128] |
| Lipopolysaccharide from Escherichia coli 055:B5 | FRET | 7.9 fM (water) 8.3 fM (serum) | Lipoglycans | [129] |
| Lipopolysaccharide from E. coli O111:B4 | Optical Colorimetric | 1 ug/mL | Lipoglycans | [130] |
| Lipopolysaccharides from E. coli | Electrochemical Amperometric | 29 ag/mL | Lipoglycans | [131] |
| Lipopolysaccharides from Escherichia Coli | Electrochemical Amperometric | 1 fg/mL | Lipoglycans | [132] |
| Lipopolysaccharides | Optical Colorimetric | 1.73 ng/mL | Lipoglycans | [133] |
| Lipopolysaccharides from Salmonella entericaserotype typhimurium, Pseudomonas aeruginosa 10 and Escherichia coli 055:B5 | Fluorescence polarization | 38.7, 88.0, and 154 ng/mL, respectively | Lipoglycans | [134] |
| Bacillus anthracis spore stimulant | Electrochemical Impedimetric | 3 × 103 CFU/mL | Spore | [135] |
| Staphylococcus aureus enterotoxin B | Fluorescence | 0.9 pg/mL | Protein | [136] |
| Staphylococcus aureus enterotoxin B | Electrochemical Impedimetric | 0.21 fM | Protein | [137] |
| Staphylococcus aureus enterotoxin B | Optical Colorimetric | 50 ng/mL | Protein | [138] |
| Staphylococcus aureus enterotoxins | Fluorescence | 1 ng/mL | Protein | [139] |
| Streptococcus mutans | Optical Colorimetric | 12 CFU/mL | Whole cell | [140] |
| Vibrio alginolyticus | Electrochemical Amperometric | 10 CFU/mL | Whole cell | [141] |
| Vibrio parahemolyticus | Optical Colorimetric | 10 CFU/mL | Whole Cell | [142] |
| Mycoplasma | Surface Enhanced Raman Scattering | 30 copies DNA/µL | Whole cell | [143] |
| Staphylococcus aureus protein A | Electrochemical Impedimetric | 10 CFU/mL | Protein | [144] |
| Staphylococcus aureus protein A | Aptamer based optical silicon biosensor | 3.17 µM | Protein | [145] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trunzo, N.E.; Hong, K.L. Recent Progress in the Identification of Aptamers Against Bacterial Origins and Their Diagnostic Applications. Int. J. Mol. Sci. 2020, 21, 5074. https://doi.org/10.3390/ijms21145074
Trunzo NE, Hong KL. Recent Progress in the Identification of Aptamers Against Bacterial Origins and Their Diagnostic Applications. International Journal of Molecular Sciences. 2020; 21(14):5074. https://doi.org/10.3390/ijms21145074
Chicago/Turabian StyleTrunzo, Nevina E., and Ka Lok Hong. 2020. "Recent Progress in the Identification of Aptamers Against Bacterial Origins and Their Diagnostic Applications" International Journal of Molecular Sciences 21, no. 14: 5074. https://doi.org/10.3390/ijms21145074