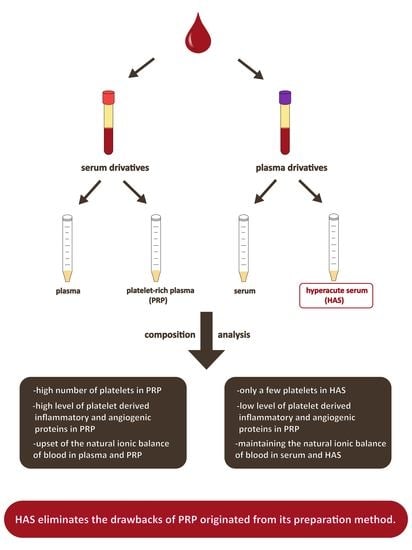
The Composition of Hyperacute Serum and Platelet-Rich Plasma Is Markedly Different despite the Similar Production Method
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Isolation of Blood Derivates
4.2. Laboratory Testing of Blood Derivates
4.3. Comprehensive Protein Analysis
4.4. Quantitative Protein Measurement by Multiplex Immunoassay and ELISA Assays
4.5. Statistical Analysis
5. Conclusions
Abbreviations
| ALCAM | activated leukocyte cell adhesion molecule |
| ALP | alkaline phosphatase |
| CD40L | cluster of differentiation 40 ligand |
| CD97 | cluster of differentiation 97 |
| CHI3L1 | chitinase 3-like protein 1 |
| CRP | C-reactive protein |
| CXCL-5 | chemokine (C-X-C motif) ligand 5 |
| C1qR1 | complement component 1 Q subcomponent receptor 1 |
| C5a | complement component 5a |
| EDTA | ethylenediaminetetraacetic acid |
| EGF | epidermal growth factor |
| EMMPRIN | extracellular matrix metalloproteinase inducer |
| ELISA | enzyme-linked immunosorbent assay |
| HAS | hyperacute serum |
| IL-17 | interleukin-17 |
| IL-1RA | interleukin-1 receptor antagonist |
| MSC | mesenchymal stem cell |
| TGF-beta | transforming growth factor beta |
| PDGF-AA | platelet-derived growth factor AA |
| PDGF-BB | platelet-derived growth factor BB |
| PRF | platelet-rich fibrin |
| PRP | platelet-rich plasma |
| SPRF | serum from platelet-rich fibrin |
| VEGF | vascular endothelial growth factor |
Supplementary Materials
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Fotouhi, A.; Maleki, A.; Dolati, S.; Aghebati-Maleki, A.; Aghebati-Maleki, L. Platelet rich plasma, stromal vascular fraction and autologous conditioned serum in treatment of knee osteoarthritis. Biomed. Pharmacother. 2018, 104, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Le, A.D.K.; Enweze, L.; DeBaun, M.R.; Dragoo, J.L. Current Clinical Recommendations for Use of Platelet-Rich Plasma. Curr. Rew. Musculoskelet. Med. 2018, 11, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Amable, P.R.; Carias, R.B.V.; Teixeira, M.V.T.; da Cruz Pacheco, I.; Corrêa do Amaral, R.J.F.; Granjeiro, J.M.; Borojevic, R. Platelet-rich plasma preparation for regenerative medicine: Optimization and quantification of cytokines and growth factors. Stem Cell Res. Ther. 2013, 4, 67. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Thakur, A. Platelet concentrates: Past, present and future. J. Oral Maxillofac. Surg. 2011, 10, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Andia, I.; Maffulli, N. Use of platelet-rich plasma for patellar tendon and medial collateral ligament injuries: Best current clinical practice. J. KNEE Surg. 2015, 28, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Masuki, H.; Okudera, T.; Watanebe, T.; Suzuki, M.; Nishiyama, K.; Okudera, H.; Nakata, K.; Uematsu, K.; Su, C.-Y.; Kawase, T. Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (A-PRF), and concentrated growth factors (CGF). Int. J. Implant. Dent. 2016, 2, 19. [Google Scholar] [CrossRef]
- El-Sharkawy, H.; Kantarci, A.; Deady, J.; Hasturk, H.; Liu, H.; Alshahat, M.; Van Dyke, T.E. Platelet-rich plasma: Growth factors and pro- and anti-inflammatory properties. J. Periodontol. 2007, 78, 661–669. [Google Scholar] [CrossRef]
- Badis, D.; Omar, B. The effectiveness of platelet-rich plasma on the skin wound healing process: A comparative experimental study in sheep. Vet. World 2018, 11, 800–808. [Google Scholar] [CrossRef]
- Lee, K.S.; Wilson, J.J.; Rabago, D.P.; Baer, G.S.; Jacobson, J.A.; Borrero, C.G. Musculoskeletal applications of platelet-rich plasma: Fad or future? AJR Am. J. Roentgenol. 2011, 196, 628–636. [Google Scholar] [CrossRef]
- Gentile, P.; Cole, J.P.; Cole, M.A.; Garcovich, S.; Bielli, A.; Scioli, M.G.; Orlandi, A.; Insalaco, C.; Cervelli, V. Evaluation of Not-Activated and Activated PRP in Hair Loss Treatment: Role of Growth Factor and Cytokine Concentrations Obtained by Different Collection Systems. Int. J. Mol. Sci. 2017, 18, 408. [Google Scholar] [CrossRef]
- Montanez-Heredia, E.; Irizar, S.; Huertas, P.J.; Otero, E.; Del Valle, M.; Prat, I.; Diaz-Gallardo, M.S.; Peran, M.; Marchal, J.A.; Hernandez-Lamas, M.D.C. Intra-Articular Injections of Platelet-Rich Plasma versus Hyaluronic Acid in the Treatment of Osteoarthritic Knee Pain: A Randomized Clinical Trial in the Context of the Spanish National Health Care System. Int. J. Mol. Sci 2016, 17, 1064. [Google Scholar] [CrossRef]
- Angelone, M.; Conti, V.; Biacca, C.; Battaglia, B.; Pecorari, L.; Piana, F.; Gnudi, G.; Leonardi, F.; Ramoni, R.; Basini, G.; et al. The Contribution of Adipose Tissue-Derived Mesenchymal Stem Cells and Platelet-Rich Plasma to the Treatment of Chronic Equine Laminitis: A Proof of Concept. Int. J. Mol. Sci 2017, 18, 2122. [Google Scholar] [CrossRef] [PubMed]
- Andia, I.; Martin, J.I.; Maffulli, N. Advances with platelet rich plasma therapies for tendon regeneration. Expert Opin. Biol. Ther. 2018, 18, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.M.N.; Pizani, N.S.; Kang, H.C.; Silva, L.A.K. Application of platelet-rich plasma in the treatment of chronic skin ulcer - case report. An. Brasileiros de Dermatologia 2014, 89, 638–640. [Google Scholar] [CrossRef]
- Shih, S. Platelet-rich plasma: Potential role in combined therapy for vitiligo. Dermatol. Ther. 2018, e12773. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.R.; Rodeo, S.; Bhutani, N.; Goodrich, L.R.; Huard, J.; Irrgang, J.; LaPrade, R.F.; Lattermann, C.; Lu, Y.; Mandelbaum, B.; et al. Optimizing Clinical Use of Biologics in Orthopaedic Surgery: Consensus Recommendations From the 2018 AAOS/NIH U-13 Conference. J. Am. Acad. Orthop. Surg. 2018, 27, e50–e63. [Google Scholar] [CrossRef] [PubMed]
- Andia, I.; Maffulli, N. A contemporary view of platelet-rich plasma therapies: Moving toward refined clinical protocols and precise indications. Regenerative Med. 2018, 13, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, C.E.; Álvarez, M.E.; Carmona, J.U. Effects of sodium citrate and acid citrate dextrose solutions on cell counts and growth factor release from equine pure-platelet rich plasma and pure-platelet rich gel. BMC Vet. Res. 2015, 11, 60. [Google Scholar] [CrossRef]
- Araki, J.; Jona, M.; Eto, H.; Aoi, N.; Kato, H.; Suga, H.; Doi, K.; Yatomi, Y.; Yoshimura, K. Optimized preparation method of platelet-concentrated plasma and noncoagulating platelet-derived factor concentrates: Maximization of platelet concentration and removal of fibrinogen. Tissue Eng. C Methods 2012, 18, 176–185. [Google Scholar] [CrossRef] [PubMed]
- do Amaral, R.J.F.C.; da Silva, N.P.; Haddad, N.F.; Lopes, L.S.; Ferreira, F.D.; Filho, R.B.; Cappelletti, P.A.; de Mello, W.; Cordeiro-Spinetti, E.; Balduino, A. Platelet-Rich Plasma Obtained with Different Anticoagulants and Their Effect on Platelet Numbers and Mesenchymal Stromal Cells Behavior In Vitro. Stem Cells Int. 2016, 2016, 11. [Google Scholar] [CrossRef]
- Schuff-Werner, P.; Steiner, M.; Fenger, S.; Gross, H.-J.; Bierlich, A.; Dreissiger, K.; Mannuß, S.; Siegert, G.; Bachem, M.; Kohlschein, P. Effective estimation of correct platelet counts in pseudothrombocytopenia using an alternative anticoagulant based on magnesium salt. Br. J. Haematol. 2013, 162, 684–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.V.; Shubhashini, N. Platelet rich fibrin: A new paradigm in periodontal regeneration. Cell Tissue Bank 2013, 14, 453–463. [Google Scholar] [CrossRef]
- Di Liddo, R.; Bertalot, T.; Borean, A.; Pirola, I.; Argentoni, A.; Schrenk, S.; Cenzi, C.; Capelli, S.; Conconi, M.T.; Parnigotto, P.P. Leucocyte and Platelet-rich Fibrin: A carrier of autologous multipotent cells for regenerative medicine. J. Cell Mol. Med. 2018, 22, 1840–1854. [Google Scholar] [CrossRef] [PubMed]
- Kardos, D.; Hornyak, I.; Simon, M.; Hinsenkamp, A.; Marschall, B.; Vardai, R.; Kallay-Menyhard, A.; Pinke, B.; Meszaros, L.; Kuten, O.; et al. Biological and Mechanical Properties of Platelet-Rich Fibrin Membranes after Thermal Manipulation and Preparation in a Single-Syringe Closed System. Int. J. Mol. Sci 2018, 19, 3433. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Aristizabal, R.F.; Lopez, C.; Alvarez, M.E.; Giraldo, C.; Prades, M.; Carmona, J.U. Long-term cytokine and growth factor release from equine platelet-rich fibrin clots obtained with two different centrifugation protocols. Cytokine 2017, 97, 149–155. [Google Scholar] [CrossRef]
- Varela, H.A.; Souza, J.C.M.; Nascimento, R.M.; Araujo, R.F., Jr.; Vasconcelos, R.C.; Cavalcante, R.S.; Guedes, P.M.; Araujo, A.A. Injectable platelet rich fibrin: Cell content, morphological, and protein characterization. Clin. Oral Investig. 2018. [Google Scholar] [CrossRef] [PubMed]
- Kapse, S.; Surana, S.; Satish, M.; Hussain, S.E.; Vyas, S.; Thakur, D. Autologous platelet-rich fibrin: Can it secure a better healing? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2018. [Google Scholar] [CrossRef]
- Wang, L.; Liu, G.; Li, Z.; Jia, B.C.; Wang, Y. Clinical application of platelet-rich fibrin in chronic wounds combined with subcutaneous stalking sinus. Zhonghua Shao Shang Za Zhi 2018, 34, 637–642. [Google Scholar]
- Shah, R.; Gowda, T.M.; Thomas, R.; Kumar, T.; Mehta, D.S. Biological activation of bone grafts using injectable platelet-rich fibrin. J. Prosthet. Dent. 2018. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Choukroun, J.; Ghanaati, S.; Miron, R.J. Behavior of Gingival Fibroblasts on Titanium Implant Surfaces in Combination with either Injectable-PRF or PRP. Int. J. Mol. Sci 2017, 18, 331. [Google Scholar] [CrossRef]
- Wong, C.C.; Kuo, T.-F.; Yang, T.-L.; Tsuang, Y.-H.; Lin, M.-F.; Chang, C.-H.; Lin, Y.-H.; Chan, W.P. Platelet-Rich Fibrin Facilitates Rabbit Meniscal Repair by Promoting Meniscocytes Proliferation, Migration, and Extracellular Matrix Synthesis. Int. J. Mol. Sci 2017, 18, 1722. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Major, B.; Vacz, G.; Kuten, O.; Hornyak, I.; Hinsenkamp, A.; Kardos, D.; Bago, M.; Cseh, D.; Sarkozi, A.; et al. The Effects of Hyperacute Serum on the Elements of the Human Subchondral Bone Marrow Niche. Stem Cells Int. 2018, 2018, 4854619. [Google Scholar] [CrossRef] [PubMed]
- Jeyakumar, V.; Niculescu-Morzsa, E.; Bauer, C.; Lacza, Z.; Nehrer, S. Platelet-Rich Plasma Supports Proliferation and Redifferentiation of Chondrocytes during In Vitro Expansion. Front. Bioeng. Biotechnol. 2017, 5, 75. [Google Scholar] [CrossRef] [PubMed]
- Kuten, O.; Simon, M.; Hornyak, I.; De Luna-Preitschopf, A.; Nehrer, S.; Lacza, Z. The Effects of Hyperacute Serum on Adipogenesis and Cell Proliferation of Mesenchymal Stromal Cells. Tissue Eng. Part A 2018, 24, 1011–1021. [Google Scholar] [CrossRef]
- Vacz, G.; Major, B.; Gaal, D.; Petrik, L.; Horvathy, D.B.; Han, W.; Holczer, T.; Simon, M.; Muir, J.M.; Hornyak, I.; et al. Hyperacute serum has markedly better regenerative efficacy than platelet-rich plasma in a human bone oxygen-glucose deprivation model. Regenerative Med. 2018, 13, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Kawase, T.; Momose, M.; Murata, M.; Saito, Y.; Suzuki, H.; Wolff, L.F.; Yoshie, H. Platelet-rich plasma contains high levels of platelet-derived growth factor and transforming growth factor-beta and modulates the proliferation of periodontally related cells in vitro. J. Periodontol. 2003, 74, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Lubkowska, A.; Dolegowska, B.; Banfi, G. Growth factor content in PRP and their applicability in medicine. J. Biol. Regul. Homeost. Agents 2012, 26, 3s–22s. [Google Scholar] [PubMed]
- Lee, J.W.; Kwon, O.H.; Kim, T.K.; Cho, Y.K.; Choi, K.Y.; Chung, H.Y.; Cho, B.C.; Yang, J.D.; Shin, J.H. Platelet-rich plasma: Quantitative assessment of growth factor levels and comparative analysis of activated and inactivated groups. Arch. Plast. Surg. 2013, 40, 530–535. [Google Scholar] [CrossRef]
- Park, H.-B.; Yang, J.-H.; Chung, K.-H. Characterization of the cytokine profile of platelet rich plasma (PRP) and PRP-induced cell proliferation and migration: Upregulation of matrix metalloproteinase-1 and -9 in HaCaT cells. Korean J. Hematol. 2011, 46, 265–273. [Google Scholar] [CrossRef]
- Andia, I.; Maffulli, N. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat. Rev. Rheumatol. 2013, 9, 721–730. [Google Scholar] [CrossRef]
- Golub, E.E.; Harrison, G.; Taylor, A.G.; Camper, S.; Shapiro, I.M. The role of alkaline phosphatase in cartilage mineralization. Bone Miner. 1992, 17, 273–278. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kardos, D.; Simon, M.; Vácz, G.; Hinsenkamp, A.; Holczer, T.; Cseh, D.; Sárközi, A.; Szenthe, K.; Bánáti, F.; Szathmary, S.; et al. The Composition of Hyperacute Serum and Platelet-Rich Plasma Is Markedly Different despite the Similar Production Method. Int. J. Mol. Sci. 2019, 20, 721. https://doi.org/10.3390/ijms20030721
Kardos D, Simon M, Vácz G, Hinsenkamp A, Holczer T, Cseh D, Sárközi A, Szenthe K, Bánáti F, Szathmary S, et al. The Composition of Hyperacute Serum and Platelet-Rich Plasma Is Markedly Different despite the Similar Production Method. International Journal of Molecular Sciences. 2019; 20(3):721. https://doi.org/10.3390/ijms20030721
Chicago/Turabian StyleKardos, Dorottya, Melinda Simon, Gabriella Vácz, Adél Hinsenkamp, Tünde Holczer, Domonkos Cseh, Adrienn Sárközi, Kálmán Szenthe, Ferenc Bánáti, Susan Szathmary, and et al. 2019. "The Composition of Hyperacute Serum and Platelet-Rich Plasma Is Markedly Different despite the Similar Production Method" International Journal of Molecular Sciences 20, no. 3: 721. https://doi.org/10.3390/ijms20030721