T Cell Proliferation Is Induced by Chronically TLR2-Stimulated Gingival Fibroblasts or Monocytes
Abstract
:1. Introduction
2. Results
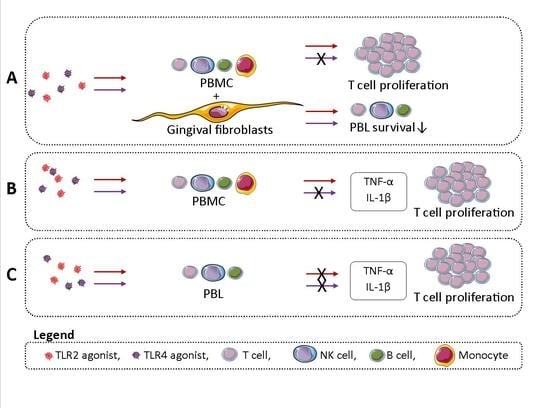
2.1. TLR Activation Reduces PBMC Survival in GF-PBMC Cocultures
2.2. TLR2 Agonists Induce T Cell Proliferation in the Presence of GF
2.3. PBMCs Express Significantly More TLR2 and TLR4 than GFs
2.4. Monocytes Play an Important Role in PBL Survival in the Absence of GFs
2.5. TLR2 Induces T Cell Proliferation in the Presence of Monocytes
2.6. TLR2 Agonists Induce Proinflammatory Cytokine Production in the Presence of Monocytes
2.7. TLR2 and TLR4 Agonists Enhance GF-Mediated T Cell Proliferation in the Absence of Monocytes
3. Discussion
3.1. TLR2 and TLR4 Responses on GFs versus Monocytes
3.2. The Role of Monocytes in TLR Activation
3.3. Proinflammatory Cytokine Production by TLR2 Activation
3.4. Effects of TLRs on T Cell Activation/Proliferation
4. Materials and Methods
4.1. Gingival Fibroblasts (GFs)
4.2. Peripheral Blood Mononuclear Cell (PBMC) Isolation
4.3. Peripheral Blood Lymphocyte (PBL) Isolation
4.4. Cultures with TLR Agonists
4.5. Cell Population Characterization with Flow Cytometry
4.6. Proliferation Assay
4.7. Gene Expression Analysis
4.8. Cytokine Production Analysis
4.9. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CD | cluster of differentiation |
| CFSE | carboxyfluorescein succinimidyl ester |
| Ct | cycle threshold |
| ELISA | enzyme-linked immunosorbent assay |
| FCS | fetal calf serum |
| GF | gingival fibroblast |
| HPRT | hypoxanthine phosphoribosyltransferase |
| IL-1β | interleukin 1 beta |
| LPS | lipopolysaccharide |
| PBL | peripheral blood lymphocyte |
| PBMC | peripheral blood mononuclear cell |
| Pg | Porphyromonas gingivalis |
| TLR | toll-like receptor |
| TNF-α | tumor necrosis alpha |
References
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef]
- Matarese, G.; Isola, G.; Anastasi, G.P.; Cutroneo, G.; Cordasco, G.; Favaloro, A.; Vita, G.; Vermiglio, G.; Milardi, D.; Zizzari, V.L.; et al. Transforming Growth Factor Beta 1 and Vascular Endothelial Growth Factor levels in the pathogenesis of periodontal disease. Eur. J. Inflamm. 2013, 11, 479–488. [Google Scholar] [CrossRef] [Green Version]
- Silva, N.; Abusleme, L.; Bravo, D.; Dutzan, N.; Garcia-Sesnich, J.; Vernal, R.; Hernandez, M.; Gamonal, J. Host response mechanisms in periodontal diseases. J. Appl. Oral Sci. 2015, 23, 329–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyle, J.; Chapple, I.L.C. Molecular aspects of the pathogenesis of periodontitis. Periodontology 2000, 69, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Beklen, A.; Hukkanen, M.; Richardson, R.; Konttinen, Y.T. Immunohistochemical localization of Toll-like receptors 1–10 in periodontitis. Oral Microbiol. Immunol. 2008, 23, 425–431. [Google Scholar] [CrossRef]
- Scheres, N.; Laine, M.L.; Sipos, P.M.; Bosch-Tijhof, C.J.; Crielaard, W.; De Vries, T.J.; Everts, V. Periodontal ligament and gingival fibroblasts from periodontitis patients are more active in interaction with Porphyromonas gingivalis. J. Periodontal Res. 2011, 46, 407–416. [Google Scholar] [CrossRef]
- Mahanonda, R.; Sa-Ard-Iam, N.; Montreekachon, P.; Pimkhaokham, A.; Yongvanichit, K.; Fukuda, M.M.; Pichyangkul, S. IL-8 and IDO Expression by Human Gingival Fibroblasts via TLRs. J. Immunol. 2007, 178, 1151–1157. [Google Scholar] [CrossRef] [Green Version]
- Massey, H.M.; Flanagan, A.M. Human osteoclasts derive from CD14-positive monocytes. Br. J. Haematol. 1999, 106, 167–170. [Google Scholar] [CrossRef]
- Dutzan, N.; Konkel, J.E.; Greenwell-Wild, T.; Moutsopoulos, N.M. Characterization of the human immune cell network at the gingival barrier. Mucosal Immunol. 2016, 9, 1163–1172. [Google Scholar] [CrossRef]
- Oberg, H.H.; Juricke, M.; Kabelitz, D.; Wesch, D. Regulation of T cell activation by TLR ligands. Eur. J. Cell Biol. 2011, 90, 582–592. [Google Scholar] [CrossRef]
- Moonen, C.G.; Alders, S.T.; Bontkes, H.J.; Schoenmaker, T.; Nicu, E.A.; Loos, B.G.; de Vries, T.J. Survival, Retention, and Selective Proliferation of Lymphocytes Is Mediated by Gingival Fibroblasts. Front. Immunol. 2018, 9, 1–18. [Google Scholar] [CrossRef] [PubMed]
- de Vries, T.J.; Schoenmaker, T.; van Veen, H.A.; Hogervorst, J.; Krawczyk, P.M.; Moonen, C.G.; Jansen, I.D. The challenge of teaching essential immunology laboratory skills to undergraduates in one month - experience on an osteoimmunology course on TLR activation. Front. Immunol. 2019, 10, 1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, B.; Sun, T.; Yu, X.H.; Yang, Y.X.; Yeo, A.E.T. The effects of TLR activation on T-cell development and differentiation. Clin. Dev. Immunol. 2012, 2012, 836485. [Google Scholar] [CrossRef] [PubMed]
- Scholzen, T.; Gerdes, J. The Ki-67 Protein: From the known to the Unknown. J. Cell. Physiol. 2000, 322, 311–322. [Google Scholar] [CrossRef]
- Ropert, C. How toll-like receptors reveal monocyte plasticity: The cutting edge of antiinflammatory therapy. Cell. Mol. Life Sci. 2018, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Donati, M. Aspects of adaptive host response in periodontitis. J. Clin. Periodontol. 2005, 32, 87–107. [Google Scholar] [CrossRef]
- Cekici, A.; Kantarci, A.; Hasturk, H.; van Dyke, T.E. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontology 2000, 64, 57–80. [Google Scholar] [CrossRef] [Green Version]
- Sokos, D.; Scheres, N.; Schoenmaker, T.; Everts, V.; de Vries, T.J. A challenge with Porphyromonas gingivalis differentially affects the osteoclastogenesis potential of periodontal ligament fibroblasts from periodontitis patients and non-periodontitis donors. J. Clin. Periodontol. 2014, 41, 95–103. [Google Scholar] [CrossRef]
- Wang, P.L.; Azuma, Y.; Shinohara, M.; Ohura, K. Toll-like receptor 4-mediated signal pathway induced by Porphyromonas gingivalis lipopolysaccharide in human gingival fibroblasts. Biochem. Biophys. Res. Commun. 2000, 273, 1161–1167. [Google Scholar] [CrossRef]
- Song, B.; Zhang, Y.L.; Chen, L.J.; Zhou, T.; Huang, W.K.; Zhou, X.; Shao, L.Q. The role of Toll-like receptors in periodontitis. Oral Dis. 2017, 23, 168–180. [Google Scholar] [CrossRef]
- Mahanonda, R.; Pichyangkul, S. Toll-like receptors and their role in periodontal health and disease. Periodontology 2000, 43, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Sojar, H.; Genco, R.J.; DeNardin, E. Intracellular signaling and cytokine induction upon interactions of Porphyromonas gingivalis fimbriae with pattern-recognition receptors. Immunol. Investig. 2004, 33, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, L. Blood monocytes and their subtypes: Established featurs and open questions. Front. Immunol. 2015, 6, 423. [Google Scholar] [CrossRef] [PubMed]
- Haziot, A.; Chen, S.; Ferrero, E.; Low, M.G.; Silber, R.; Goyert, S.M. The monocyte differentiation antigen, CD14, is anchored to the cell membrane by a phosphatidylinositol linkage. J. Immunol. 1988, 141, 547–552. [Google Scholar] [PubMed]
- Watanabe, A.; Takeshita, A.; Kitano, S.; Hanazawa, S. CD14-mediated signal pathway of Porphyromonas gingivalis lipopolysaccharide in human gingival fibroblasts. Infect. Immun. 1996, 64, 4488–4494. [Google Scholar]
- Bautista-Hernández, L.A.; Gómez-Olivares, J.L.; Buentello-Volante, B.; Lucio, V.M. Fibroblasts: The unknown sentinels eliciting immune responses against microorganisms. Eur. J. Microbiol. Immunol. 2017, 7, 151–157. [Google Scholar] [CrossRef]
- Liu, J.; Liu, W.; Xie, Y.; Wang, Y.; Ouyang, X. Adhesion of monocytes to periodontal fibroblasts requires activation of NOD1/2- and TLR4-mediated LFA-1 and VLA-4. Arch. Oral Biol. 2015, 60, 834–844. [Google Scholar] [CrossRef]
- Ospelt, C.; Gay, S. TLRs and chronic inflammation. Int. J. Biochem. Cell Biol. 2010, 42, 495–505. [Google Scholar] [CrossRef] [Green Version]
- Bryn, T.; Yaqub, S.; Mahic, M.; Henjum, K.; Aandahl, E.M.; Tasén, K. LPS-activated monocytes suppress T-cell immune responses and induce FOXP3+ T cells through a COX-2-PGE2-dependent mechanism. Int. Immunol. 2008, 20, 235–245. [Google Scholar] [CrossRef] [Green Version]
- de Vries, T.J.; Yousovich, J.; Schoenmaker, T.; Scheres, N.; Everts, V. Tumor necrosis factor alpha antagonist infliximab inhibits osteoclast formation of peripheral blood mononuclear cells but does not affect periodontal ligament fibroblast-mediated osteoclast formation. J. Periodontal Res. 2016, 51, 186–195. [Google Scholar] [CrossRef]
- Li, X.; Jiang, S.; Tapping, R.I. Toll-like receptor signaling in cell proliferation and survival. Cytokine 2010, 49, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nambu, A.; Nakae, S.; Iwakura, Y. IL-1β, but not IL-1α, is required for antigen-specific T cell activation and the induction of local inflammation in the delayed-type hypersensitivity responses. Int. Immunol. 2006, 18, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.K.; Gracias, D.T.; Croft, M. TNF activity and T cells. Cytokine 2018, 101, 14–18. [Google Scholar] [CrossRef] [PubMed]







| Gene | Primer Sequence | |
|---|---|---|
| TLR2 | Forward Reverse | GGCTTCTCTGTCTTGTGACCG GAGCCCTGAGGGAATGGAG |
| TLR4 | Forward Reverse | CTGCAATGGATCAAGGAACCAG CCATTCGTTCAACTTCCACCA |
| KI67 | Forward Reverse | CGAGACGCCTGGTTACTATCAA GGATACGGATGTCACATTCAATACC |
| HPRT | Forward Reverse | TGACCTTGATTTATTTTGCATACC CGAGCAAGACGTTCAGTCCT |
| Antibody | Fluorochrome | Vendor/Cat No./Clone | Laser Lines | Emission Filters |
|---|---|---|---|---|
| Anti-human CD56 | PE | eBiosciences by Thermo Fisher Scientific/#12056742/MSSB | 488 nm | 586/42 |
| Anti-human CD3 | BV506 | eBiosciences by Thermo Fisher Scientific/#563109/UCHT1 | 405 nm | 528/45 |
| Anti-human CD19 | APC | eBiosciences by Thermo Fisher Scientific/#17019842/SJ25C1 | 640 nm | 660/10 |
| Anti-human CD14 | Efluor 450 | BD biosciences /#48014942/61D3 | 405 nm | 448/45 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moonen, C.G.J.; Karlis, G.D.; Schoenmaker, T.; Forouzanfar, T.; Loos, B.G.; de Vries, T.J. T Cell Proliferation Is Induced by Chronically TLR2-Stimulated Gingival Fibroblasts or Monocytes. Int. J. Mol. Sci. 2019, 20, 6134. https://doi.org/10.3390/ijms20246134
Moonen CGJ, Karlis GD, Schoenmaker T, Forouzanfar T, Loos BG, de Vries TJ. T Cell Proliferation Is Induced by Chronically TLR2-Stimulated Gingival Fibroblasts or Monocytes. International Journal of Molecular Sciences. 2019; 20(24):6134. https://doi.org/10.3390/ijms20246134
Chicago/Turabian StyleMoonen, Carolyn G. J., Gerasimos D. Karlis, Ton Schoenmaker, Tim Forouzanfar, Bruno G. Loos, and Teun J. de Vries. 2019. "T Cell Proliferation Is Induced by Chronically TLR2-Stimulated Gingival Fibroblasts or Monocytes" International Journal of Molecular Sciences 20, no. 24: 6134. https://doi.org/10.3390/ijms20246134