The Effect of Schisandra chinensis Baillon on Cross-Talk between Oxidative Stress, Endoplasmic Reticulum Stress, and Mitochondrial Signaling Pathway in Testes of Varicocele-Induced SD Rat
Abstract
:1. Introduction
2. Results
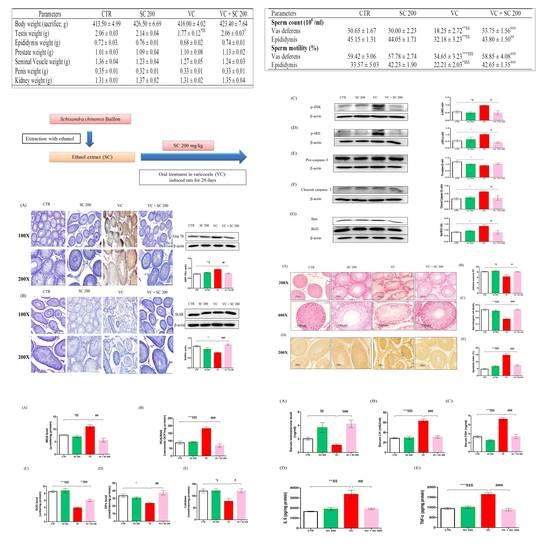
2.1. Effects of SC on Body and Reproductive Organ Weights of VC-Induced Rats
2.2. Effects of SC on Sperm Parameters of VC-Induced Rats
2.3. Effect of SC on Sperm Motility in Human Semen Sample
2.4. SC Alleviates VC-Induced Testicular Histopathological Damage and Germ Cell Apoptosis
2.5. SC Reduces VC-Induced Oxidative Stress in Testes
2.6. Effect of SC on VC-Induced Hormone Level and Anti-Inflammatory Activity
2.7. ER Stress Inhibition by SC Decreases VC-Induced Germ Cell Apoptosis in Rat Testes
2.8. Effect of SC on VC-Induced Mitochondria-Dependent Apoptotic Pathway
2.9. Effect of SC on StAR Protein of VC-Induced Rats
3. Discussion
4. Materials and Methods
4.1. Plant Material and Extract Preparation
4.2. Identification of Major Compounds
4.3. Animals and Experimental Design
4.4. Chemicals and Reagents
4.5. Assessment of Sperm Count and Sperm Motility
4.6. Measurements of Hormones Levels
4.7. Histopathology and Terminal Deoxynucleotidyl Transferase-Mediated (dUTP) Nick-End Labeling (TUNEL) Staining of Testis
4.8. Immunohistochemical Staining of GRP-78 and StAR Expression
4.9. Malondialdehyde (MDA) and Reactive Oxygen Species (ROS)/Reactive Nitrogen Species (RNS) Level
4.10. Evaluation of Antioxidant Enzyme’s Activity
4.11. Assessment of Inflammatory Biomarkers
4.12. Western Blot Analysis
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CTR | control |
| VC | varicocele |
| SC | Schisandra chinensis |
| LH | luteinizing hormone |
| FSH | follicle stimulating hormone |
| WBC | white blood cell |
| RBC | red blood cell |
| Hb | hemoglobin |
| Hct | hematocrit |
| AST | aspartate aminotransferase |
| ALT | alanine aminotransferase |
| p.o. | per oral |
| ANOVA | analysis of variance |
| SEM | standard error of the mean |
| ER | Endoplasmic reticulum |
| ROS/RNS | Reactive oxygen species/reactive nitrogen species |
| MDA | Malondialdehyde |
| SOD | Superoxide dismutase |
| GPx | Glutathione peroxidase |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor necrosis factor-α |
| GRP-78 | Glucose-regulated protein-78 |
| p-JNK | Phosphorylated c-Jun-N-terminal kinase |
| p-IRE1α | Phosphorylated Inositol-Requiring Transmembrane Kinase/Endoribonuclease 1α |
| JNK | C-jun-N-terminal kinase |
| Bax | BCL 2 associated X protein |
| Bcl-2 | B-cell lymphoma 2 |
| StAR | Steroidogenic acute regulatory protein. |
References
- Jensen, C.F.S.; Ostergren, P.; Dupree, J.M.; Ohl, D.A.; Sonksen, J.; Fode, M. Varicocele and male infertility. Nat. Rev. Urol. 2017, 14, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Mendes, T.B.; Paccola, C.C.; de Oliveira Neves, F.M.; Simas, J.N.; da Costa Vaz, A.; Cabral, R.E.; Vendramini, V.; Miraglia, S.M. Resveratrol improves reproductive parameters of adult rats varicocelized in peripuberty. Reproduction 2016, 152, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Zheng, X.M.; Li, S.W.; Yang, Z.W.; Hu, L.Q. Effects of epidermal growth factor on sperm content and motility of rats with surgically induced varicoceles. Asian J. Androl. 2006, 8, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, M.I.; Koca, O.; Keles, M.O.; Haklar, G.; Baykan, O.; Ercan, F.; Tok, O.E.; Karaman, M.I. The impact of unilateral experimental rat varicocele model on testicular histopathology, Leydig cell counts, and intratesticular testosterone levels of both testes. Urol. J. 2013, 10, 973–980. [Google Scholar]
- Sahin, Z.; Celik-Ozenci, C.; Akkoyunlu, G.; Korgun, E.T.; Acar, N.; Erdogru, T.; Demir, R.; Ustunel, I. Increased expression of interleukin-1alpha and interleukin-1beta is associated with experimental varicocele. Fertil. Steril. 2006, 85, 1265–1275. [Google Scholar] [CrossRef]
- Tek, M.; Cayan, S.; Yilmaz, N.; Oguz, I.; Erdem, E.; Akbay, E. The effect of vascular endothelial growth factor on spermatogenesis and apoptosis in experimentally varicocele-induced adolescent rats. Fertil. Steril. 2009, 91, 2247–2252. [Google Scholar] [CrossRef]
- Soni, K.K.; Zhang, L.T.; Choi, B.R.; Karna, K.K.; You, J.H.; Shin, Y.S.; Lee, S.W.; Kim, C.Y.; Zhao, C.; Chae, H.J.; et al. Protective effect of MOTILIPERM in varicocele-induced oxidative injury in rat testis by activating phosphorylated inositol requiring kinase 1alpha (p-IRE1alpha) and phosphorylated c-Jun N-terminal kinase (p-JNK) pathways. Pharm. Biol. 2018, 56, 94–103. [Google Scholar] [CrossRef]
- Will, M.A.; Swain, J.; Fode, M.; Sonksen, J.; Christman, G.M.; Ohl, D. The great debate: Varicocele treatment and impact on fertility. Fertil. Steril. 2011, 95, 841–852. [Google Scholar] [CrossRef]
- Moshtaghion, S.M.; Malekinejad, H.; Razi, M.; Shafie-Irannejad, V. Silymarin protects from varicocele-induced damages in testis and improves sperm quality: Evidence for E2f1 involvement. Syst. Biol. Reprod. Med. 2013, 59, 270–280. [Google Scholar] [CrossRef]
- Cao, S.S.; Kaufman, R.J. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid. Redox Signal. 2014, 21, 396–413. [Google Scholar] [CrossRef]
- Cocuzza, M.; Agarwal, A. Nonsurgical treatment of male infertility: Specific and empiric therapy. Biologics 2007, 1, 259–269. [Google Scholar] [PubMed]
- Tian, R.H.; Ma, M.; Zhu, Y.; Yang, S.; Wang, Z.Q.; Zhang, Z.S.; Wan, C.F.; Li, P.; Liu, Y.F.; Wang, J.L.; et al. Effects of aescin on testicular repairment in rats with experimentally induced varicocele. Andrologia 2014, 46, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, D.F. Analysis of Schisandra chinensis and Schisandra sphenanthera. J. Chromatogr. A 2009, 1216, 1980–1990. [Google Scholar] [CrossRef] [PubMed]
- Szopa, A.; Ekiert, R.; Ekiert, H. Current knowledge of Schisandra chinensis (Turcz.) Baill. (Chinese magnolia vine) as a medicinal plant species: A review on the bioactive components, pharmacological properties, analytical and biotechnological studies. Phytochem. Rev. 2017, 16, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Wikman, G. Pharmacology of Schisandra chinensis Bail.: An overview of Russian research and uses in medicine. J. Ethnopharmacol. 2008, 118, 183–212. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Li, M.; Jeung, E.B.; Lee, G.S.; Hong, E.J.; Choi, Y.W.; An, B.S. Therapeutic effects of Schisandra chinensis on the hyperprolactinemia in rat. Int. J. Oncol. 2017, 50, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.R.; Kim, H.K.; Park, J.K. Effects of Schisandra chinensis fruit extract and gomisin A on the contractility of penile corpus cavernosum smooth muscle: A potential mechanism through the nitric oxide—Cyclic guanosine monophosphate pathway. Nutr. Res. Pract. 2018, 12, 291–297. [Google Scholar] [CrossRef]
- Huang, H.; Shen, Z.; Geng, Q.; Wu, Z.; Shi, P.; Miao, X. Protective effect of Schisandra chinensis bee pollen extract on liver and kidney injury induced by cisplatin in rats. Biomed. Pharmacother. 2017, 95, 1765–1776. [Google Scholar] [CrossRef]
- Ko, K.M.; Chiu, P.Y. Biochemical basis of the “Qi-invigorating” action of Schisandra berry (wu-wei-zi) in Chinese medicine. Am. J. Chin. Med. 2006, 34, 171–176. [Google Scholar] [CrossRef]
- Bae, H.; Kim, R.; Kim, Y.; Lee, E.; Jin Kim, H.; Pyo Jang, Y.; Jung, S.K.; Kim, J. Effects of Schisandra chinensis Baillon (Schizandraceae) on lipopolysaccharide induced lung inflammation in mice. J. Ethnopharmacol. 2012, 142, 41–47. [Google Scholar] [CrossRef]
- Kim, J.S.; Takanche, J.S.; Kim, J.E.; Jeong, S.H.; Han, S.H.; Yi, H.K. Schisandra chinensis extract ameliorates age-related muscle wasting and bone loss in ovariectomized rats. Phytother. Res. 2019, 33, 1865–1877. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shen, N.; Qi, L.; Chen, W.; Dong, Z.; Zhao, D.H. Efficacy of Schizandra chinesis polysaccharide on cyclophosphamide induced dyszoospermia of rats and its effects on reproductive hormones. Zhongguo Zhong Xi Yi Jie He Za Zhi 2013, 33, 361–364. [Google Scholar] [PubMed]
- Karna, K.K.; Shin, Y.S.; Choi, B.R.; Kim, H.K.; Park, J.K. The Role of Endoplasmic Reticulum Stress Response in Male Reproductive Physiology and Pathology: A Review. World J. Mens. Health 2019, 37. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.K.; Nam, J.S.; Kim, J.H.; Yun, Y.R.; Han, C.W.; Kim, B.J.; Jeong, H.S.; Ha, K.T.; Jung, M.H. Schisandra chinensis extract ameliorates nonalcoholic fatty liver via inhibition of endoplasmic reticulum stress. J. Ethnopharmacol. 2016, 185, 96–104. [Google Scholar] [CrossRef]
- Li, Y.Z.; Ren, S.; Yan, X.T.; Li, H.P.; Li, W.; Zheng, B.; Wang, Z.; Liu, Y.Y. Improvement of Cisplatin-induced renal dysfunction by Schisandra chinensis stems via anti-inflammation and anti-apoptosis effects. J. Ethnopharmacol. 2018, 217, 228–237. [Google Scholar] [CrossRef]
- Karna, K.K.; Choi, B.R.; You, J.H.; Shin, Y.S.; Soni, K.K.; Cui, W.S.; Lee, S.W.; Kim, C.Y.; Kim, H.K.; Park, J.K. Cross-talk between ER stress and mitochondrial pathway mediated adriamycin-induced testicular toxicity and DA-9401 modulate adriamycin-induced apoptosis in Sprague-Dawley rats. Cancer Cell Int. 2019, 19, 85. [Google Scholar] [CrossRef]
- Rashtbari, H.; Razi, M.; Hassani-Bafrani, H.; Najaran, H. Berberine reinforces Sertoli cells niche and accelerates spermatogonial stem cells renewal in experimentally-induced varicocele condition in rats. Phytomedicine 2018, 40, 68–78. [Google Scholar] [CrossRef]
- Celik, O.; Kutlu, O.; Tekcan, M.; Celik-Ozenci, C.; Koksal, I.T. Role of TNF-related apoptosis-inducing ligand (TRAIL) in the pathogenesis of varicocele-induced testicular dysfunction. Asian J. Androl. 2013, 15, 269–274. [Google Scholar] [CrossRef]
- Park, H.J.; Koo, Y.K.; Park, M.J.; Hwang, Y.K.; Hwang, S.Y.; Park, N.C. Restoration of Spermatogenesis Using a New Combined Herbal Formula of Epimedium koreanum Nakai and Angelica gigas Nakai in an Luteinizing Hormone-Releasing Hormone Agonist-Induced Rat Model of Male Infertility. World J. Mens. Health 2017, 35, 170–177. [Google Scholar] [CrossRef]
- Blevrakis, E.; Chatzidarellis, E.; Anyfantakis, D.; Sakellaris, G.; Raissaki, M.; Zoras, O.; Mamoulakis, C.; Sofras, F.; Chrysos, E. Impact of varicocele on biological markers of gonadal function. Hernia 2016, 20, 435–439. [Google Scholar] [CrossRef]
- Asadi, N.; Kheradmand, A.; Gholami, M.; Saidi, S.H.; Mirhadi, S.A. Effect of royal jelly on testicular antioxidant enzymes activity, MDA level and spermatogenesis in rat experimental Varicocele model. Tissue Cell 2019, 57, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Bressler, R.S.; Lustbader, I.J. Effect of testosterone on development of the lumen in seminiferous tubules of the rat. Andrologia 1978, 10, 291–298. [Google Scholar] [PubMed]
- Panner Selvam, M.K.; Agarwal, A.; Sharma, R.; Samanta, L.; Gupta, S.; Dias, T.R.; Martins, A.D. Protein Fingerprinting of Seminal Plasma Reveals Dysregulation of Exosome-Associated Proteins in Infertile Men with Unilateral Varicocele. World J. Mens Health 2019. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Lee, S.H.; Choi, H.W.; Lee, H.S.; Lee, J.S.; Seo, J.T. Abnormal Human Sperm Parameters Contribute to Sperm DNA Fragmentation in Men with Varicocele. World J. Mens. Health 2018, 36, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.I.; Do, G.M.; Lee, H.M.; Ok, H.M.; Shin, J.H.; Kwon, O. Schisandra Chinensis Baillon regulates the gene expression of phase II antioxidant/detoxifying enzymes in hepatic damage induced rats. Nutr. Res. Pract. 2014, 8, 272–277. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Rana, M.; Qiu, E.; AlBunni, H.; Bui, A.D.; Henkel, R. Role of oxidative stress, infection and inflammation in male infertility. Andrologia 2018, 50, 13126. [Google Scholar] [CrossRef]
- Habibi, B.; Seifi, B.; Mougahi, S.M.; Ojaghi, M.; Sadeghipour, H.R. Increases in interleukin-6 and interferon-gamma levels is progressive in immature rats with varicocele. Ir. J. Med. Sci. 2015, 184, 531–537. [Google Scholar] [CrossRef]
- Hedger, M.P. Immunophysiology and pathology of inflammation in the testis and epididymis. J. Androl. 2011, 32, 625–640. [Google Scholar] [CrossRef]
- Kang, Y.S.; Han, M.H.; Hong, S.H.; Park, C.; Hwang, H.J.; Kim, B.W.; Kyoung, K.H.; Choi, Y.W.; Kim, C.M.; Choi, Y.H. Anti-inflammatory Effects of Schisandra chinensis (Turcz.) Baill Fruit Through the Inactivation of Nuclear Factor-kappaB and Mitogen-activated Protein Kinases Signaling Pathways in Lipopolysaccharide-stimulated Murine Macrophages. J. Cancer Prev. 2014, 19, 279–287. [Google Scholar] [CrossRef]
- Luo, D.Y.; Yang, G.; Liu, J.J.; Yang, Y.R.; Dong, Q. Effects of varicocele on testosterone, apoptosis and expression of StAR mRNA in rat Leydig cells. Asian J. Androl. 2011, 13, 287–291. [Google Scholar] [CrossRef] [Green Version]
- Manna, P.R.; Stetson, C.L.; Slominski, A.T.; Pruitt, K. Role of the steroidogenic acute regulatory protein in health and disease. Endocrine 2016, 51, 7–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzel, E.; Arlier, S.; Guzeloglu-Kayisli, O.; Tabak, M.S.; Ekiz, T.; Semerci, N.; Larsen, K.; Schatz, F.; Lockwood, C.J.; Kayisli, U.A. Endoplasmic Reticulum Stress and Homeostasis in Reproductive Physiology and Pathology. Int. J. Mol. Sci. 2017, 18, 792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huo, R.; Zhu, Y.F.; Ma, X.; Lin, M.; Zhou, Z.M.; Sha, J.H. Differential expression of glucose-regulated protein 78 during spermatogenesis. Cell Tissue Res. 2004, 316, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Soni, K.K.; Kim, H.K.; Choi, B.R.; Karna, K.K.; You, J.H.; Cha, J.S.; Shin, Y.S.; Lee, S.W.; Kim, C.Y.; Park, J.K. Dose-dependent effects of cisplatin on the severity of testicular injury in Sprague Dawley rats: Reactive oxygen species and endoplasmic reticulum stress. Drug Des. Dev. Ther. 2016, 10, 3959–3968. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.D.; Lee, T.H.; Cheng, W.H.; Jeng, S.Y. Involved intrinsic apoptotic pathway of testicular tissues in varicocele-induced rats. World J. Urol. 2009, 27, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Onur, R.; Semercioz, A.; Orhan, I.; Yekeler, H. The effects of melatonin and the antioxidant defence system on apoptosis regulator proteins (Bax and Bcl-2) in experimentally induced varicocele. Urol. Res. 2004, 32, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.Z.; Rao, T.; Cheng, F.; Yu, W.M.; Ruan, Y.; Yuan, R.; Zhu, S.M.; Du, Y.; Xiao, C.C. Effect of varicocelectomy treatment on spermatogenesis and apoptosis via the induction of heat shock protein 70 in varicoceleinduced rats. Mol. Med. Rep. 2017, 16, 5406–5412. [Google Scholar] [CrossRef] [Green Version]
- Ghafarizadeh, A.A.; Vaezi, G.; Shariatzadeh, M.A.; Malekirad, A.A. Effect of in vitro selenium supplementation on sperm quality in asthenoteratozoospermic men. Andrologia 2018, 50. [Google Scholar] [CrossRef]
- Chae, M.R.; Kang, S.J.; Lee, K.P.; Choi, B.R.; Kim, H.K.; Park, J.K.; Kim, C.Y.; Lee, S.W. Onion (Allium cepa L.) peel extract (OPE) regulates human sperm motility via protein kinase C-mediated activation of the human voltage-gated proton channel. Andrology 2017, 5, 979–989. [Google Scholar] [CrossRef] [Green Version]
- Soni, K.K.; Shin, Y.S.; Choi, B.R.; Karna, K.K.; Kim, H.K.; Lee, S.W.; Kim, C.Y.; Park, J.K. Protective effect of DA-9401 in finasteride-induced apoptosis in rat testis: Inositol requiring kinase 1 and c-Jun N-terminal kinase pathway. Drug Des. Dev. Ther. 2017, 11, 2969–2979. [Google Scholar] [CrossRef] [Green Version]






| Parameters | CTR | SC 200 | VC | VC + SC 200 |
|---|---|---|---|---|
| Body weight (sacrifice; g) | 413.50 ± 4.99 | 426.50 ± 6.69 | 416.00 ± 4.02 | 423.40 ± 7.64 |
| Testis weight (g) | 2.06 ± 0.03 | 2.14 ± 0.04 | 1.77 ± 0.12 *$$ | 2.06 ± 0.03 # |
| Epididymis weight (g) | 0.72 ± 0.03. | 0.76 ± 0.01 | 0.68 ± 0.02 | 0.74 ± 0.01 |
| Prostate weight (g) | 1.01 ± 0.03 | 1.09 ± 0.04 | 1.10 ± 0.08 | 1.13 ± 0.02 |
| Seminal Vesicle weight (g) | 1.36 ± 0.04 | 1.23 ± 0.04 | 1.27 ± 0.05 | 1.24 ± 0.03 |
| Penis weight (g) | 0.35 ± 0.01 | 0.32 ± 0.01 | 0.33 ± 0.01 | 0.33 ± 0.01 |
| Kidney weight (g) | 1.31 ± 0.01 | 1.37 ± 0.02 | 1.31 ± 0.02 | 1.35 ± 0.04 |
| Parameters | CTR | SC 200 | VC | VC + SC 200 |
|---|---|---|---|---|
| Sperm count (106/mL) | ||||
| Vas deferens | 30.65 ± 1.67 | 30.00 ± 2.23 | 18.25 ± 2.72 **$$ | 33.75 ± 1.56 ### |
| Epididymis | 45.15 ± 1.31 | 44.05 ± 1.71 | 32.18 ± 3.23 **$$ | 43.80 ± 1.50 ## |
| Sperm motility (%) | ||||
| Vas deferens | 59.42 ± 3.06 | 57.78 ± 2.74 | 34.65 ± 3.23 ***$$$ | 58.85 ± 4.08 ### |
| Epididymis | 33.57 ± 5.03 | 42.23 ± 1.90 | 22.21 ± 2.03 *$$$ | 42.65 ± 1.35 ### |
| Group | Zero | 3 h | Increase in Sperm Motility (%) | |||
|---|---|---|---|---|---|---|
| Count (106/mL) | Motility (%) | Count (106/mL) | Motility (%) | |||
| Patient 1 | Control | 38 | 57.8 | 32 | 50.0 | –13.5 |
| SC 0.05 mg/mL | 38 | 50.0 | 32 | 66.5 | 33.0 | |
| Patient 2 | Control | 62 | 67.0 | 58 | 65.7 | –1.9 |
| SC 0.05 mg/mL | 66 | 64.2 | 54 | 74.5 | 16.0 | |
| Patient 3 | Control | 31 | 51.6 | 29 | 38.0 | –26.4 |
| SC 0.05 mg/mL | 34 | 45.8 | 23 | 48.5 | 5.9 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karna, K.K.; Choi, B.R.; Kim, M.-J.; Kim, H.K.; Park, J.K. The Effect of Schisandra chinensis Baillon on Cross-Talk between Oxidative Stress, Endoplasmic Reticulum Stress, and Mitochondrial Signaling Pathway in Testes of Varicocele-Induced SD Rat. Int. J. Mol. Sci. 2019, 20, 5785. https://doi.org/10.3390/ijms20225785
Karna KK, Choi BR, Kim M-J, Kim HK, Park JK. The Effect of Schisandra chinensis Baillon on Cross-Talk between Oxidative Stress, Endoplasmic Reticulum Stress, and Mitochondrial Signaling Pathway in Testes of Varicocele-Induced SD Rat. International Journal of Molecular Sciences. 2019; 20(22):5785. https://doi.org/10.3390/ijms20225785
Chicago/Turabian StyleKarna, Keshab Kumar, Bo Ram Choi, Min-Ji Kim, Hye Kyung Kim, and Jong Kwan Park. 2019. "The Effect of Schisandra chinensis Baillon on Cross-Talk between Oxidative Stress, Endoplasmic Reticulum Stress, and Mitochondrial Signaling Pathway in Testes of Varicocele-Induced SD Rat" International Journal of Molecular Sciences 20, no. 22: 5785. https://doi.org/10.3390/ijms20225785