Bursting at the Seams: Molecular Mechanisms Mediating Astrocyte Swelling
Abstract
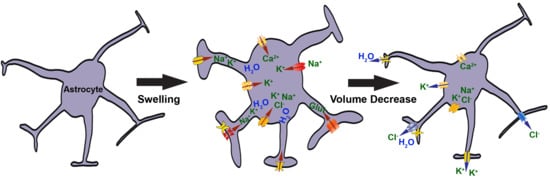
:1. Introduction
2. Techniques for Assessing Astrocyte Swelling
2.1. In vitro Studies
2.2. In vivo and In Situ Histological Studies
3. Implication of Astrocyte Swelling in Disease
4. Molecular Mechanisms of Astrocyte Swelling
4.1. Gap Junction Channels
4.2. AQP4
4.3. TRPV4
4.4. Kir4.1
4.5. Na+/K+-ATPase and NKCC
4.6. SUR1-TRPM4
5. Regulatory Volume Decrease and Volume Regulated Anion Channels
6. Two Molecules That Induce Astrocyte Swelling—Glutamate and Ammonia
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lin, S.-H.; Bergles, D. Physiological characteristics of NG2-expressing glial cells. J. Neurocytol. 2002, 31, 537–549. [Google Scholar] [CrossRef]
- Orkand, R.; Nicholls, J.; Kuffler, S. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J. Neurophysiol. 1966, 29, 788–806. [Google Scholar] [CrossRef] [PubMed]
- Holthoff, K.; Witte, O. Directed spatial potassium redistribution in rat neocortex. Glia 2000, 29, 288–292. [Google Scholar] [CrossRef] [Green Version]
- Nagelhus, E.; Mathiisen, T.; Ottersen, O. Aquaporin-4 in the central nervous system: Cellular and subcellular distribution and coexpression with KIR4.1. Neuroscience 2004, 129, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Stokum, J.A.; Mehta, R.I.; Ivanova, S.; Yu, E.; Gerzanich, V.; Simard, J.M. Heterogeneity of aquaporin-4 localization and expression after focal cerebral ischemia underlies differences in white versus grey matter swelling. Acta Neuropathol. Commun. 2015, 3, 1–61. [Google Scholar] [CrossRef] [PubMed]
- Nakada, T.; Kwee, I.L. Fluid Dynamics Inside the Brain Barrier. Neuroscientist 2018. [Google Scholar] [CrossRef]
- Kimelberg, H. Swelling and Volume Control in Brain Astroglial Cells. In Advances in Comparative and Environmental Physiology; Springer: Berlin, Germany, 1991; Volume 9, pp. 81–117. [Google Scholar]
- Scemes, E.; Spray, D. Increased intercellular communication in mouse astrocytes exposed to hyposmotic shocks. Glia 1998, 24, 74–84. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Barres, B. Astrocyte heterogeneity: An underappreciated topic in neurobiology. Curr. Opin. Neurobiol. 2010, 20, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Pekny, M.; Wilhelmsson, U.; Tatlisumak, T.; Pekna, M. Astrocyte activation and reactive gliosis-A new target in stroke? Neurosci. Lett. 2019, 689, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Oberheim, N.; Goldman, S.; Nedergaard, M. Heterogeneity of astrocytic form and function. In Methods in Molecular Biology; Humana Press, Springer: Berlin/Heidelberg, Germany, 2012; Volume 814, pp. 23–45. [Google Scholar]
- Benesova, J.; Rusnakova, V.; Honsa, P.; Pivonkova, H.; Dzamba, D.; Kubista, M.; Anderova, M. Distinct expression/function of potassium and chloride channels contributes to the diverse volume regulation in cortical astrocytes of GFAP/EGFP mice. PLoS ONE 2012, 7, e29725. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, W.; Lu, Y.; Lu, Y.; Xu, L.; Fang, Q.; Wu, M.; Jia, M.; Wang, Y.; Dong, L.; et al. Aquaporin 4-Mediated Glutamate-Induced Astrocyte Swelling Is Partially Mediated through Metabotropic Glutamate Receptor 5 Activation. Front. Cell. Neurosci. 2017, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Benesova, J.; Hock, M.; Butenko, O.; Prajerova, I.; Anderova, M.; Chvatal, A. Quantification of astrocyte volume changes during ischemia in situ reveals two populations of astrocytes in the cortex of GFAP/EGFP mice. J. Neurosci. Res. 2009, 87, 96–111. [Google Scholar] [CrossRef]
- Shi, Z.; Lixin, X.; Yi, L.; Dong, L.; Xu, Y.; Shaohua, Y.; Fang, Y. Comparison of the differences in glutamate-induced astrocyte swelling between Wistar and Sprague-Dawley rats. Acta Lab. Anim. Sci. Sin. 2016, 24, 454–459. [Google Scholar]
- Duverger, D.; MacKenzie, E.T. The Quantification of Cerebral Infarction following Focal Ischemia in the Rat: Influence of Strain, Arterial Pressure, Blood Glucose Concentration, and Age. J. Cereb. Blood Flow Metab. 1987, 8, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmsson, U.; Li, L.; Pekna, M.; Berthold, C.-H.H.; Blom, S.; Eliasson, C.; Renner, O.; Bushong, E.; Ellisman, M.; Morgan, T.E.; et al. Absence of glial fibrillary acidic protein and vimentin prevents hypertrophy of astrocytic processes and improves post-traumatic regeneration. J. Neurosci. 2004, 24, 5016–5021. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Kintner, D.B.; Flagella, M.; Shull, G.E.; Sun, D. Astrocytes from Na(+)-K(+)-Cl(−) cotransporter-null mice exhibit absence of swelling and decrease in EAA release. Am. J. Physiol. Cell Physiol. 2002, 282, C1147–C1160. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Kintner, D.B.; Sun, D. Contribution of Na(+)-K(+)-Cl(−) cotransporter to high-[K(+)](o)- induced swelling and EAA release in astrocytes. Am. J. Physiol. Cell Physiol. 2002, 282, C1136–C1146. [Google Scholar] [CrossRef]
- Vardjan, N.; Horvat, A.; Anderson, J.E.; Yu, D.; Croom, D.; Zeng, X.; Lužnik, Z.; Kreft, M.; Teng, Y.D.; Kirov, S.A.; et al. Adrenergic activation attenuates astrocyte swelling induced by hypotonicity and neurotrauma. Glia 2016, 64, 1034–1049. [Google Scholar] [CrossRef]
- Lisjak, M.; Potokar, M.; Rituper, B.; Jorgačevski, J.; Zorec, R. AQP4e-Based Orthogonal Arrays Regulate Rapid Cell Volume Changes in Astrocytes. J. Neurosci. 2017, 37, 10748–10756. [Google Scholar] [CrossRef]
- Mola, M.; Sparaneo, A.; Gargano, C.; Spray, D.C.; Svelto, M.; Frigeri, A.; Scemes, E.; Nicchia, G. The speed of swelling kinetics modulates cell volume regulation and calcium signaling in astrocytes: A different point of view on the role of aquaporins. Glia 2016, 64, 139–154. [Google Scholar] [CrossRef]
- Stokum, J.A.; Kwon, M.S.; Woo, S.K.; Tsymbalyuk, O.; Vennekens, R.; Gerzanich, V.; Simard, J.M. SUR1-TRPM4 and AQP4 form a heteromultimeric complex that amplifies ion/water osmotic coupling and drives astrocyte swelling. Glia 2018, 66, 108–125. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Gao, K.; Zhang, H.; Takeda, M.; Yao, J. Suppression of cell membrane permeability by suramin: Involvement of its inhibitory actions on connexin 43 hemichannels. Br. J. Pharmacol. 2014, 171, 3448–3462. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, A.; Tong, X.; Ospel, J. Norenberg Role of cerebral endothelial cells in the astrocyte swelling and brain edema associated with acute hepatic encephalopathy. Neuroscience 2012, 218, 305–316. [Google Scholar] [CrossRef]
- Jayakumar, A.R.; Tong, X.Y.; Ruiz-Cordero, R.; Bregy, A.; Bethea, J.R.; Bramlett, H.M.; Norenberg, M.D. Activation of NF-κB Mediates Astrocyte Swelling and Brain Edema in Traumatic Brain Injury. J. Neurotraum 2014, 31, 1249–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, K.V.R.; Chen, M.; Simard, J.M.; Norenberg, M.D. Increased aquaporin-4 expression in ammonia-treated cultured astrocytes. Neuroreport 2003, 14, 2379–2382. [Google Scholar]
- O’Connor, E.; Kimelberg, H. Role of calcium in astrocyte volume regulation and in the release of ions and amino acids. J. Neurosci. 1993, 13, 2638–2650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsen, B.R.; Assentoft, M.; Cotrina, M.L.; Hua, S.Z.; Nedergaard, M.; Kaila, K.; Voipio, J.; MacAulay, N. Contributions of the Na+/K+-ATPase, NKCC1, and Kir4.1 to hippocampal K+ clearance and volume responses. Glia 2014, 62, 608–622. [Google Scholar] [CrossRef]
- Pagrsic, T.; Potokar, M.; Haydon, P.; Zorec, R.; Kreft, M. Astrocyte swelling leads to membrane unfolding, not membrane insertion. J. Neurochem. 2006, 99, 514–523. [Google Scholar] [CrossRef] [Green Version]
- Kimelberg, H.; Rutledge, E.; Goderie, S.; Charniga, C. Astrocytic swelling due to hypotonic or high K+ medium causes inhibition of glutamate and aspartate uptake and increases their release. J. Cereb. Blood Flow Metab. 1995, 15, 409–416. [Google Scholar] [CrossRef]
- Abdullaev, I.F.; Rudkouskaya, A.; Schools, G.P.; Kimelberg, H.K.; Mongin, A.A. Pharmacological comparison of swelling-activated excitatory amino acid release and Cl-currents in cultured rat astrocytes. J. Physiol. 2006, 572, 677–689. [Google Scholar] [CrossRef]
- Dibaj, P.; Kaiser, M.; Hirrlinger, J.; Kirchhoff, F.; Neusch, C. Kir4.1 channels regulate swelling of astroglial processes in experimental spinal cord edema. J. Neurochem. 2007, 103, 2620–2628. [Google Scholar] [CrossRef]
- Anderova, M.; Benesova, J.; Mikesova, M.; Dzamba, D.; Honsa, P.; Kriska, J.; Butenko, O.; Novosadova, V.; Valihrach, L.; Kubista, M.; et al. Altered astrocytic swelling in the cortex of α-syntrophin-negative GFAP/EGFP mice. PLoS ONE 2014, 9, e113444. [Google Scholar] [CrossRef]
- Rakers, C.; Schmid, M.; Petzold, G.C. TRPV4 channels contribute to calcium transients in astrocytes and neurons during peri-infarct depolarizations in a stroke model. Glia 2017, 65, 1550–1561. [Google Scholar] [CrossRef]
- Lee, J.; Han, Y.-E.; Favorov, O.; Tommerdahl, M.; Whitsel, B.; Lee, J.C. Fluoride Induces a Volume Reduction in CA1 Hippocampal Slices via MAP Kinase Pathway Through Volume Regulated Anion Channels. Exp. Neurobiol. 2016, 25, 72–78. [Google Scholar] [CrossRef]
- Larsen, B.R.; MacAulay, N. Activity-dependent astrocyte swelling is mediated by pH-regulating mechanisms. Glia 2017, 65, 1668–1681. [Google Scholar] [CrossRef]
- Gorse, K.M.; Lantzy, M.; Lee, E.D.; Lafrenaye, A.D. Transient Receptor Potential Melastatin 4 Induces Astrocyte Swelling but Not Death after Diffuse Traumatic Brain Injury. J. Neurotraum 2018, 35, 1694–1704. [Google Scholar] [CrossRef]
- Sullivan, S.M.; Björkman, T.S.; Miller, S.M.; Colditz, P.B.; Pow, D.V. Morphological changes in white matter astrocytes in response to hypoxia/ischemia in the neonatal pig. Brain Res. 2010, 1319, 164–174. [Google Scholar] [CrossRef]
- Simard, J.M.; Kent, T.A.; Chen, M.; Tarasov, K.V.; Gerzanich, V. Brain oedema in focal ischaemia: Molecular pathophysiology and theoretical implications. Lancet Neurol. 2007, 6, 258–268. [Google Scholar] [CrossRef]
- Stokum, J.A.; Kurland, D.B.; Gerzanich, V.; Simard, J.M. Mechanisms of Astrocyte-Mediated Cerebral Edema. Neurochem. Res. 2015, 40, 317–328. [Google Scholar] [CrossRef]
- Murphy, T.R.; Binder, D.K.; Fiacco, T.A. Turning down the volume: Astrocyte volume change in the generation and termination of epileptic seizures. Neurobiol. Dis. 2017, 104, 24–32. [Google Scholar] [CrossRef]
- Norenberg, M.; Rao, R.K.; Jayakumar, A. Mechanisms of Ammonia-Induced Astrocyte Swelling. Metab. Brain Dis. 2005, 20, 303–318. [Google Scholar] [CrossRef]
- Dudek, F.E.; Obenaus, A.; Tasker, J.G. Osmolality-induced changes in extracellular volume alter epileptiform bursts independent of chemical synapses in the rat: Importance of non-synaptic mechanisms in hippocampal epileptogenesis. Neurosci. Lett. 1990, 120, 267–270. [Google Scholar] [CrossRef]
- Roper, S.N.; Obenaus, A.; Dudek, E.F. Osmolality and nonsynaptic epileptiform bursts in rat CA1 and dentate gyrus. Ann. Neurol. 1992, 31, 81–85. [Google Scholar] [CrossRef]
- Rosen, A.S.; Andrew, R.D. Osmotic effects upon excitability in rat neocortical slices. Neuroscience 1990, 38, 579–590. [Google Scholar] [CrossRef]
- Saly, V.; Andrew, R. CA3 neuron excitation and epileptiform discharge are sensitive to osmolality. J. Neurophysiol. 1993, 69, 2200–2208. [Google Scholar] [CrossRef]
- Traynelis, S.; Dingledine, R. Role of extracellular space in hyperosmotic suppression of potassium-induced electrographic seizures. J. Neurophysiol. 1989, 61, 927–938. [Google Scholar] [CrossRef]
- Kimelberg, H.; Goderie, S.; Higman, S.; Pang, S.; Waniewski, R. Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J. Neurosci. 1990, 10, 1583–1591. [Google Scholar] [CrossRef] [Green Version]
- Schober, A.L.; Mongin, A.A. Intracellular levels of glutamate in swollen astrocytes are preserved via neurotransmitter reuptake and de novo synthesis: Implications for hyponatremia. J. Neurochem. 2015, 135, 176–185. [Google Scholar] [CrossRef]
- Unterberg, A.W.; Stover, J.; Kress, B.; Kiening, K.L. Edema and brain trauma. Neuroscience 2004, 129, 1019–1027. [Google Scholar] [CrossRef]
- Jayakumar, A.R.; Norenberg, M.D. The Na–K–Cl Co-transporter in astrocyte swelling. Metab. Brain Dis. 2010, 25, 31–38. [Google Scholar] [CrossRef]
- Min, R.; van der Knaap, M.S. Genetic defects disrupting glial ion and water homeostasis in the brain. Brain Pathol. 2018, 28, 372–387. [Google Scholar] [CrossRef]
- Rash, J. Molecular disruptions of the panglial syncytium block potassium siphoning and axonal saltatory conduction: Pertinence to neuromyelitis optica and other demyelinating diseases of the central nervous system. Neuroscience 2010, 168, 982–1008. [Google Scholar] [CrossRef]
- Nagy, J.; Rash, J. Connexins and gap junctions of astrocytes and oligodendrocytes in the CNS. Brain Res. Rev. 2000, 32, 29–44. [Google Scholar] [CrossRef]
- Nagy, J.; Dudek, F.; Rash, J. Update on connexins and gap junctions in neurons and glia in the mammalian nervous system. Brain Res. Rev. 2004, 47, 191–215. [Google Scholar] [CrossRef]
- O’Carroll, S.J.; Alkadhi, M.; Nicholson, L.F.; Green, C.R. Connexin 43 mimetic peptides reduce swelling, astrogliosis, and neuronal cell death after spinal cord injury. Cell Commun. Adhes. 2008, 15, 27–42. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, L.; Zhang, L.; Chen, B.; Yang, L.; Li, X.; Li, Y.; Yu, H. Inhibition of Connexin 43 Hemichannels Alleviates Cerebral Ischemia/Reperfusion Injury via the TLR4 Signaling Pathway. Front. Cell. Neurosci. 2018. [Google Scholar] [CrossRef]
- Lutz, S.E.; Zhao, Y.; Gulinello, M.; Lee, S.C.; Raine, C.S.; Brosnan, C.F. Deletion of astrocyte connexins 43 and 30 leads to a dysmyelinating phenotype and hippocampal CA1 vacuolation. J. Neurosci. 2009, 29, 7743–7752. [Google Scholar] [CrossRef]
- Ezan, P.; André, P.; Cisternino, S.; Saubaméa, B.; Boulay, A.-C.; Doutremer, S.; Thomas, M.-A.; Quenech’du, N.; Giaume, C.; Cohen-Salmon, M. Deletion of Astroglial Connexins Weakens the Blood–Brain Barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1457–1467. [Google Scholar] [CrossRef] [Green Version]
- Castejón, O. Electron microscopy of astrocyte changes and subtypes in traumatic human edematous cerebral cortex: A review. Ultrastruct. Pathol. 2013, 37, 417–424. [Google Scholar] [CrossRef]
- Huang, C.; Han, X.; Li, X.; Lam, E.; Peng, W.; Lou, N.; Torres, A.; Yang, M.; Garre, J.; Tian, G.-F.; et al. Critical Role of Connexin 43 in Secondary Expansion of Traumatic Spinal Cord Injury. J. Neurosci. 2012, 32, 3333–3338. [Google Scholar] [CrossRef] [Green Version]
- Matthias, K.; Kirchhoff, F.; Seifert, G.; Hüttmann, K.; Matyash, M.; Kettenmann, H.; Steinhäuser, C. Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J. Neurosci. 2003, 23, 1750–1758. [Google Scholar] [CrossRef] [PubMed]
- Wallraff, A.; Odermatt, B.; Willecke, K.; Steinhäuser, C. Distinct types of astroglial cells in the hippocampus differ in gap junction coupling. Glia 2004, 48, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Badaut, J.; Ashwal, S.; Obenaus, A. Aquaporins in Cerebrovascular Disease: A Target for Treatment of Brain Edema. Cerebrovasc. Dis. 2011, 31, 521–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badaut, J.; Fukuda, A.M.; Jullienne, A.; Petry, K.G. Aquaporin and brain diseases. Biochim. Biophys. Acta 2014, 1840, 1554–1565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuda, A.M.; Badaut, J. Aquaporin 4: A player in cerebral edema and neuroinflammation. J. Neuroinflamm. 2012, 9, 279. [Google Scholar] [CrossRef] [PubMed]
- Clément, T.; Rodriguez-Grande, B.; Badaut, J. Aquaporins in brain edema. J. Neurosci. Res. 2018. [Google Scholar] [CrossRef]
- Yang, X.; Ransom, B.R.; Ma, J.-F.F. The role of AQP4 in neuromyelitis optica: More answers, more questions. J. Neuroimmunol. 2016, 298, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Nagelhus, E.; Amiry-Moghaddam, M.; Bourque, C.; Agre, P.; Ottersen, O. Specialized Membrane Domains for Water Transport in Glial Cells: High-Resolution Immunogold Cytochemistry of Aquaporin-4 in Rat Brain. J. Neurosci. 1997, 17, 171–180. [Google Scholar] [CrossRef]
- Hirt, L.; Price, M.; Mastour, N.; Brunet, J.-F.F.; Barrière, G.; Friscourt, F.; Badaut, J. Increase of aquaporin 9 expression in astrocytes participates in astrogliosis. J. Neurosci. Res. 2018, 96, 194–206. [Google Scholar] [CrossRef]
- Moe, S.; Sorbo, J.; Sogaard, R.; Zeuthen, T.; Petter Ottersen, O.; Holen, T. New isoforms of rat aquaporin-4. Genomics 2008, 91, 367–377. [Google Scholar] [CrossRef]
- Lman, M.; Kitchen, P.; Woodroofe, M.; Brown, J.E.; Bill, R.M.; Conner, A.C.; Conner, M.T. Hypothermia increases aquaporin 4 (AQP4) plasma membrane abundance in human primary cortical astrocytes via a calcium/transient receptor potential vanilloid 4 (TRPV4)- and calmodulin-mediated mechanism. Eur. J. Neurosci. 2017, 46, 2542–2547. [Google Scholar]
- Amiry-Moghaddam, M.; Xue, R.; Haug, F.-M.M.; Neely, J.D.; Bhardwaj, A.; Agre, P.; Adams, M.E.; Froehner, S.C.; Mori, S.; Ottersen, O.P. Alpha-syntrophin deletion removes the perivascular but not endothelial pool of aquaporin-4 at the blood-brain barrier and delays the development of brain edema in an experimental model of acute hyponatremia. FASEB J. 2004, 18, 542–544. [Google Scholar] [CrossRef] [PubMed]
- Thrane, A.S.; Rappold, P.M.; Fujita, T.; Torres, A.; Bekar, L.K.; Takano, T.; Peng, W.; Wang, F.; Thrane, V.; Enger, R.; et al. Critical role of aquaporin-4 (AQP4) in astrocytic Ca2+ signaling events elicited by cerebral edema. Proc. Natl. Acad. Sci. USA 2011, 108, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Strohschein, S.; Hüttmann, K.; Gabriel, S.; Binder, D.K.; Heinemann, U.; Steinhäuser, C. Impact of aquaporin-4 channels on K+ buffering and gap junction coupling in the hippocampus. Glia 2011, 59, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, A.M.; Adami, A.; Pop, V.; Bellone, J.A.; Coats, J.S.; Hartman, R.E.; Ashwal, S.; Obenaus, A.; Badaut, J. Posttraumatic Reduction of Edema with Aquaporin-4 RNA Interference Improves Acute and Chronic Functional Recovery. J. Cereb. Blood Flow Metab. 2013, 33, 1621–1632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haj-Yasein, N.; Jensen, V.; Østby, I.; Omholt, S.W.; Voipio, J.; Kaila, K.; Ottersen, O.P.; Hvalby, Ø.; Nagelhus, E.A. Aquaporin-4 regulates extracellular space volume dynamics during high-frequency synaptic stimulation: A gene deletion study in mouse hippocampus. Glia 2012, 60, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Sato, J.; Horibe, S.; Kawauchi, S.; Sasaki, N.; Hirata, K.; Rikitake, Y. Involvement of aquaporin-4 in laminin-enhanced process formation of mouse astrocytes in 2D culture: Roles of dystroglycan and α-syntrophin in aquaporin-4 expression. J. Neurochem. 2018, 147, 495–513. [Google Scholar] [CrossRef] [PubMed]
- Frydenlund, D.S.; Bhardwaj, A.; Otsuka, T.; Mylonakou, M.N.; Yasumura, T.; Davidson, K.G.; Zeynalov, E.; Skare, Ø.; Laake, P.; Haug, F.-M.; et al. Temporary loss of perivascular aquaporin-4 in neocortex after transient middle cerebral artery occlusion in mice. Proc. Natl. Acad. Sci. USA 2006, 103, 13532–13536. [Google Scholar] [CrossRef] [Green Version]
- Steiner, E.; Enzmann, G.U.; Lin, S.; Ghavampour, S.; Hannocks, M.; Zuber, B.; Rüegg, M.A.; Sorokin, L.; Engelhardt, B. Loss of astrocyte polarization upon transient focal brain ischemia as a possible mechanism to counteract early edema formation. Glia 2012, 60, 1646–1659. [Google Scholar] [CrossRef]
- Schliess, F.; Sinning, R.; Fischer, R.; Schmalenbach, C.; Häussinger, D. Calcium-dependent activation of Erk-1 and Erk-2 after hypo-osmotic astrocyte swelling. Biochem. J. 1996, 320 Pt 1, 167–171. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, H.; Davis, J.B.; Smart, D.; Jerman, J.C.; Smith, G.D.; Hayes, P.; Vriens, J.; Cairns, W.; Wissenbach, U.; Prenen, J.; et al. Activation of TRPV4 Channels (hVRL-2/mTRP12) by Phorbol Derivatives. J. Biol. Chem. 2002, 277, 13569–13577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramsey, I.; Delling, M.; Clapham, D.E. An introduction to TRP channels. Ann. Rev. Physiol. 2006, 68, 619–647. [Google Scholar] [CrossRef] [PubMed]
- Benfenati, V.; Amiry-Moghaddam, M.; Caprini, M.; Mylonakou, M.; Rapisarda, C.; Ottersen, O.; Ferroni, S. Expression and functional characterization of transient receptor potential vanilloid-related channel 4 (TRPV4) in rat cortical astrocytes. Neuroscience 2007, 148, 876–892. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, A.; Brignone, M.S.; Molinari, P.; Visentin, S.; Nuccio, C.; Macchia, G.; Aiello, C.; Bertini, E.; Aloisi, F.; Petrucci, T.C.; et al. Megalencephalic leukoencephalopathy with subcortical cysts protein 1 functionally cooperates with the TRPV4 cation channel to activate the response of astrocytes to osmotic stress: Dysregulation by pathological mutations. Hum. Mol. Genet. 2012, 21, 2166–2180. [Google Scholar] [CrossRef] [PubMed]
- Butenko, O.; Dzamba, D.; Benesova, J.; Honsa, P.; Benfenati, V.; Rusnakova, V.; Ferroni, S.; Anderova, M. The increased activity of TRPV4 channel in the astrocytes of the adult rat hippocampus after cerebral hypoxia/ischemia. PLoS ONE 2012, 7, e39959. [Google Scholar] [CrossRef]
- Shi, M.; Du, F.; Liu, Y.; Li, L.; Cai, J.; Zhang, G.-F.; Xu, X.-F.; Lin, T.; Cheng, H.-R.; Liu, X.-D.; et al. Glial cell-expressed mechanosensitive channel TRPV4 mediates infrasound-induced neuronal impairment. Acta Neuropathol. 2013, 126, 725–739. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, W.; Friedman, J.M. Abnormal osmotic regulation in trpv4-/- mice. Proc. Natl. Acad. Sci. USA 2003, 100, 13698–13703. [Google Scholar] [CrossRef] [Green Version]
- Liedtke, W.; Choe, Y.; Martí-Renom, M.; Bell, A.; Denis, C.; Sali, A.; Hudspeth, A.; Friedman, J.; Heller, S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 2000, 103, 525–535. [Google Scholar] [CrossRef]
- Suzuki, M.; Mizuno, A.; Kodaira, K.; Imai, M. Impaired pressure sensation in mice lacking TRPV4. J. Biol. Chem. 2003, 278, 22664–22668. [Google Scholar] [CrossRef]
- Vriens, J.; Watanabe, H.; Janssens, A.; Droogmans, G.; Voets, T.; Nilius, B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc. Natl. Acad. Sci. USA 2004, 101, 396–401. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, H.; Tian, W.; Yoshida, K.; Roullet, J.-B.; Cohen, D.M. Regulation of a TRP channel by tyrosine phosphorylation: Src family kinase-dependent phosphorylation of TRPV4 on Y253 mediates its response to hypotonic stress. J. Biol. Chem. 2003, 278, 11520–11527. [Google Scholar] [CrossRef]
- Watanabe, H.; Vriens, J.; Prenen, J.; Droogmans, G.; Voets, T.; Nilius, B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 2003, 424, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Benfenati, V.; Caprini, M.; Dovizio, M.; Mylonakou, M.N.; Ferroni, S.; Ottersen, O.P.; Amiry-Moghaddam, M. An aquaporin-4/transient receptor potential vanilloid 4 (AQP4/TRPV4) complex is essential for cell-volume control in astrocytes. Proc. Natl. Acad. Sci. USA 2011, 108, 2563–2568. [Google Scholar] [CrossRef] [PubMed]
- Nwaobi, S.E.; Cuddapah, V.A.; Patterson, K.C.; Randolph, A.C.; Olsen, M.L. The role of glial-specific Kir4.1 in normal and pathological states of the CNS. Acta Neuropathol. 2016, 132, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Seifert, G.; Hüttmann, K.; Binder, D.K.; Hartmann, C.; Wyczynski, A.; Neusch, C.; Steinhäuser, C. Analysis of Astroglial K+ Channel Expression in the Developing Hippocampus Reveals a Predominant Role of the Kir4.1 Subunit. J. Neurosci. 2009, 29, 7474–7488. [Google Scholar] [CrossRef] [Green Version]
- Djukic, B.; Casper, K.B.; Philpot, B.D.; Chin, L.-S.; McCarthy, K.D. Conditional Knock-Out of Kir4.1 Leads to Glial Membrane Depolarization, Inhibition of Potassium and Glutamate Uptake, and Enhanced Short-Term Synaptic Potentiation. J. Neurosci. 2007, 27, 11354–11365. [Google Scholar] [CrossRef] [Green Version]
- Trimmer, J.S. Subcellular Localization of K+ Channels in Mammalian Brain Neurons: Remarkable Precision in the Midst of Extraordinary Complexity. Neuron 2015, 85, 238–256. [Google Scholar] [CrossRef] [Green Version]
- Haj-Yasein, N.; Jensen, V.; Vindedal, G.; Gundersen, G.; Klungland, A.; Ottersen, O.; Hvalby, Ø.; Nagelhus, E. Evidence that compromised K+ spatial buffering contributes to the epileptogenic effect of mutations in the human kir4.1 gene (KCNJ10). Glia 2011, 59, 1635–1642. [Google Scholar] [CrossRef] [Green Version]
- Kofuji, P.; Newman, E.A. Potassium buffering in the central nervous system. Neuroscience 2004, 129, 1043–1054. [Google Scholar] [CrossRef] [Green Version]
- D’Ambrosio, R.; Gordon, D.S.; Winn, H. Differential role of KIR channel and Na(+)/K(+)-pump in the regulation of extracellular K(+) in rat hippocampus. J. Neurophysiol. 2002, 87, 87–102. [Google Scholar] [CrossRef]
- Ransom, C.B.; Ransom, B.R.; Sontheimer, H. Activity-dependent extracellular K + accumulation in rat optic nerve: The role of glial and axonal Na + pumps. J. Physiol. 2000, 522, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Pannicke, T.; Iandiev, I.; Uckermann, O.; Biedermann, B.; Kutzera, F.; Wiedemann, P.; Wolburg, H.; Reichenbach, A.; Bringmann, A. A potassium channel-linked mechanism of glial cell swelling in the postischemic retina. Mol. Cell. Neurosci. 2004, 26, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Obara-Michlewska, M.; Pannicke, T.; Karl, A.; Bringmann, A.; Reichenbach, A.; Szeliga, M.; Hilgier, W.; Wrzosek, A.; Szewczyk, A.; Albrecht, J. Down-regulation of Kir4.1 in the cerebral cortex of rats with liver failure and in cultured astrocytes treated with glutamine: Implications for astrocytic dysfunction in hepatic encephalopathy. J. Neurosci. Res. 2011, 89, 2018–2027. [Google Scholar] [CrossRef] [PubMed]
- Milton, M.; Smith, P.D. It’s All about Timing: The Involvement of Kir4.1 Channel Regulation in Acute Ischemic Stroke Pathology. Front. Cell. Neurosci. 2018, 12, 36. [Google Scholar] [CrossRef]
- Hoppe, D.; Kettenmann, H. Carrier-mediated Cl− transport in cultured mouse oligodendrocytes. J. Neurosci. Res. 1989, 23, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Dempsey, R.J.; Sun, D. Na+-K+-Cl− Cotransporter in Rat Focal Cerebral Ischemia. J. Cereb. Blood Flow Metab. 2001, 21, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yan, Y.; Kintner, D.B.; Lytle, C.; Sun, D. GABA-Mediated Trophic Effect on Oligodendrocytes Requires Na-K-2Cl Cotransport Activity. J. Neurophysiol. 2003, 90, 1257–1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasantes-Morales, H.; Vázquez-Juárez, E. Transporters and Channels in Cytotoxic Astrocyte Swelling. Neurochem. Res. 2012, 37, 2379–2387. [Google Scholar] [CrossRef]
- Staub, F.; Soffel, M.; Berger, S.; Eriskat, J.; Baethmann, A. Treatment of vasogenic brain edema with the novel Cl- transport inhibitor torasemide. J. Neurotrauma 1994, 11, 679–690. [Google Scholar] [CrossRef]
- Bardutzky, J.; Shen, Q.; Henninger, N.; Bouley, J.; Duong, T.Q.; Fisher, M. Differences in ischemic lesion evolution in different rat strains using diffusion and perfusion imaging. Stroke 2005, 36, 2000–2005. [Google Scholar] [CrossRef]
- Chen, M.; Simard, J.M. Cell swelling and a nonselective cation channel regulated by internal Ca2+ and ATP in native reactive astrocytes from adult rat brain. J. Neurosci. 2001, 21, 6512–6521. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Dong, Y.; Simard, J.M. Functional coupling between sulfonylurea receptor type 1 and a nonselective cation channel in reactive astrocytes from adult rat brain. J. Neurosci. 2003, 23, 8568–8577. [Google Scholar] [CrossRef] [PubMed]
- Simard, J.M.; Chen, M.; Tarasov, K.V.; Bhatta, S.; Ivanova, S.; Melnitchenko, L.; Tsymbalyuk, N.; West, A.G.; Gerzanich, V. Newly expressed SUR1-regulated NCCa-ATP channel mediates cerebral edema after ischemic stroke. Nat. Med. 2006, 12, 433–440. [Google Scholar] [CrossRef] [Green Version]
- Mehta, R.I.; Tosun, C.; Ivanova, S.; Tsymbalyuk, N.; Famakin, B.M.; Kwon, M.; Castellani, R.J.; Gerzanich, V.; Simard, J.M. Sur1-Trpm4 Cation Channel Expression in Human Cerebral Infarcts. J. Neuropathol. Exp. Neurol. 2015, 74, 835–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vennekens, R.; Nilius, B. Transient Receptor Potential (TRP) Channels. Handb. Exp. Pharmacol. 2007, 179, 269–285. [Google Scholar]
- Woo, S.; Kwon, M.; Ivanov, A.; Gerzanich, V.; Simard, J.M. The Sulfonylurea Receptor 1 (Sur1)-Transient Receptor Potential Melastatin 4 (Trpm4) Channel*. J. Biol. Chem. 2013, 288, 3655–3667. [Google Scholar] [CrossRef]
- Zerangue, N.; Schwappach, B.; Jan, Y.; Jan, L. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K(ATP) channels. Neuron 1999, 22, 537–548. [Google Scholar] [CrossRef]
- Sharma, N.; Crane, A.; Clement, J.; Gonzalez, G.; Babenko, A.; Bryan, J.; Aguilar-Bryan, L. The C terminus of SUR1 is required for trafficking of KATP channels. J. Biol. Chem. 1999, 274, 20628–20632. [Google Scholar] [CrossRef] [PubMed]
- Sheth, K.N.; Elm, J.J.; Molyneaux, B.J.; Hinson, H.; Beslow, L.A.; Sze, G.K.; Ostwaldt, A.-C.; del Zoppo, G.J.; Simard, J.M.; Jacobson, S.; et al. Safety and efficacy of intravenous glyburide on brain swelling after large hemispheric infarction (GAMES-RP): A randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2016, 15, 1160–1169. [Google Scholar] [CrossRef]
- Kimelberg, H.K.; Frangakis, M.V. Furosemide-and bumetanide-sensitive ion transport and volume control in primary astrocyte cultures from rat brain. Brain Res. 1985, 361, 125–134. [Google Scholar] [CrossRef]
- Olson, J.E.; Alexander, C.; Feller, D.; Clayman, M.; Ramnath, E. Hypoosmotic volume regulation of astrocytes in elevated extracellular potassium. J. Neurosci. Res. 1995, 40, 333–342. [Google Scholar] [CrossRef]
- Vitarella, D.; DiRisio, D.J.; Kimelberg, H.K.; Aschner, M. Potassium and Taurine Release Are Highly Correlated with Regulatory Volume Decrease in Neonatal Primary Rat Astrocyte Cultures. J. Neurochem. 1994, 63, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, P.S.; Nilsson, M.; Wågberg, M.; Rönnbäck, L.; Hansson, E. Volume regulation of single astroglial cells in primary culture. Neurosci. Lett. 1992, 143, 195–199. [Google Scholar] [CrossRef]
- Pekny, M.; Nilsson, M. Astrocyte activation and reactive gliosis. Glia 2005, 50, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Gründer, S.; Thiemann, A.; Pusch, M.; Jentsch, T. Regions involved in the opening of ClC-2 chloride channel by voltage and cell volume. Nature 1992, 360, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Bi, M.M.; Hong, S.; Zhou, H.Y.; Wang, H.W.; Wang, L.N.; Zheng, Y.J. Chloride channelopathies of ClC-2. Int. J. Mol. Sci. 2013, 15, 218–249. [Google Scholar] [CrossRef]
- Makara, J.K.; Rappert, A.; Matthias, K.; Steinhäuser, C.; Spät, A.; Kettenmann, H. Astrocytes from mouse brain slices express ClC-2-mediated Cl− currents regulated during development and after injury. Mol. Cell. Neurosci. 2003, 23, 521–530. [Google Scholar] [CrossRef]
- Parkerson, K.A.; Sontheimer, H. Biophysical and pharmacological characterization of hypotonically activated chloride currents in cortical astrocytes. Glia 2004, 46, 419–436. [Google Scholar] [CrossRef] [Green Version]
- Blanz, J.; Schweizer, M.; Auberson, M.; Maier, H.; Muenscher, A.; Hübner, C.A.; Jentsch, T.J. Leukoencephalopathy upon Disruption of the Chloride Channel ClC-2. J. Neurosci. 2007, 27, 6581–6589. [Google Scholar] [CrossRef] [Green Version]
- Depienne, C.; Bugiani, M.; Dupuits, C.; Galanaud, D.; Touitou, V.; Postma, N.; van Berkel, C.; Polder, E.; Tollard, E.; Darios, F.; et al. Brain white matter oedema due to ClC-2 chloride channel deficiency: An observational analytical study. Lancet Neurol. 2013, 12, 659–668. [Google Scholar] [CrossRef]
- Benfenati, V.; Nicchia, G.P.; Svelto, M.; Rapisarda, C.; Frigeri, A.; Ferroni, S. Functional down-regulation of volume-regulated anion channels in AQP4 knockdown cultured rat cortical astrocytes. J. Neurochem. 2007, 100, 87–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, Z.; Dubin, A.E.; Mathur, J.; Tu, B.; Reddy, K.; Miraglia, L.J.; Reinhardt, J.; Orth, A.P.; Patapoutian, A. SWELL1, a Plasma Membrane Protein, Is an Essential Component of Volume-Regulated Anion Channel. Cell 2014, 157, 447–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voss, F.K.; Ullrich, F.; Münch, J.; Lazarow, K.; Lutter, D.; Mah, N.; Andrade-Navarro, M.A.; von Kries, J.P.; Stauber, T.; Jentsch, T.J. Identification of LRRC8 Heteromers as an Essential Component of the Volume-Regulated Anion Channel VRAC. Science 2014, 344, 634–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Formaggio, F.; Saracino, E.; Mola, M.G.; Rao, S.B.; Amiry-Moghaddam, M.; Muccini, M.; Zamboni, R.; Nicchia, G.P.; Caprini, M.; Benfenati, V. LRRC8A is essential for swelling-activated chloride current and for regulatory volume decrease in astrocytes. FASEB J. 2019, 33, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Syeda, R.; Qiu, Z.; Dubin, A.E.; Murthy, S.E.; Florendo, M.N.; Mason, D.E.; Mathur, J.; Cahalan, S.M.; Peters, E.C.; Montal, M.; et al. LRRC8 Proteins Form Volume-Regulated Anion Channels that Sense Ionic Strength. Cell 2016, 164, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Ishibashi, T.; Okamoto, T.; Matsuda, T.; Hashimoto, H.; Baba, A. Transient treatments with L-glutamate and threo-beta-hydroxyaspartate induce swelling of rat cultured astrocytes. Neurochem. Int. 2000, 36, 167–173. [Google Scholar] [CrossRef]
- MacAulay, N.; Gether, U.; Klaerke, D.; Zeuthen, T. Water transport by the human Na+-coupled glutamate cotransporter expressed in Xenopus oocytes. J. Physiol. 2001, 530, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Schneider, G.; Baethmann, A.; Kempski, O. Mechanisms of glial swelling induced by glutamate. Can. J. Physiol. Pharmacol. 1992, 70, S334–S343. [Google Scholar] [CrossRef]
- Jakubovicz, D.; Klip, A. Lactic acid-induced swelling in C6 glial cells via Na+/H+ exchange. Brain Res. 1989, 485, 215–224. [Google Scholar] [CrossRef]
- Liang, D.; Bhatta, S.; Gerzanich, V.; Simard, J.M. Cytotoxic edema: Mechanisms of pathological cell swelling. Neurosurg. Focus 2007, 22, E2. [Google Scholar] [CrossRef]
- Jayakumar, A.; Valdes, V.; Tong, X.; Shamaladevi, N.; Gonzalez, W. Norenberg Sulfonylurea receptor 1 contributes to the astrocyte swelling and brain edema in acute liver failure. Transl. Stroke Res. 2014, 5, 28–37. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lafrenaye, A.D.; Simard, J.M. Bursting at the Seams: Molecular Mechanisms Mediating Astrocyte Swelling. Int. J. Mol. Sci. 2019, 20, 330. https://doi.org/10.3390/ijms20020330
Lafrenaye AD, Simard JM. Bursting at the Seams: Molecular Mechanisms Mediating Astrocyte Swelling. International Journal of Molecular Sciences. 2019; 20(2):330. https://doi.org/10.3390/ijms20020330
Chicago/Turabian StyleLafrenaye, Audrey D., and J. Marc Simard. 2019. "Bursting at the Seams: Molecular Mechanisms Mediating Astrocyte Swelling" International Journal of Molecular Sciences 20, no. 2: 330. https://doi.org/10.3390/ijms20020330