The Effect of Polyphenols on Hypercholesterolemia through Inhibiting the Transport and Expression of Niemann–Pick C1-Like 1
Abstract
:1. Identification of Cholesterol Transporter
2. Meaning of Inhibition of NPC1L1
3. NPC1L1 Transport Kinetic Parameters
4. NPC1L1-Mediated Anti-Dyslipidemia Effect of Polyphenols
5. Luteolin Inhibit NPC1L1 Transport in the Caco-2 Cell Monolayer
6. Luteolin and Quercetin Decreased the Blood Cholesterol Level in Rats
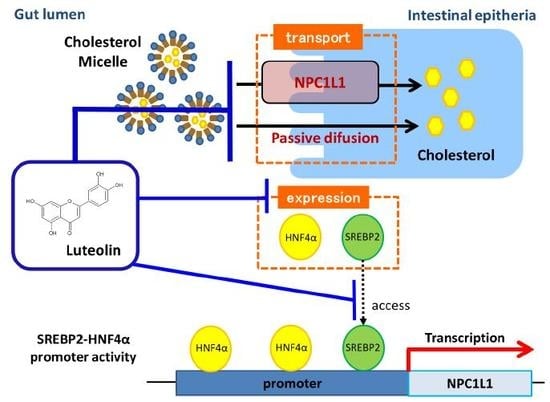
7. The Effect of Luteolin on NPC1L1 Expression
8. The Effect of Luteolin on the Activity of the NPC1L1 Promoter
9. The Effect of Luteolin on Hypercholesterolemia, Excluding the Effect on NPC1L1
10. Conclusions and Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | aminotransferase |
| AP | adaptor protein |
| APOB | apolipoprotein B |
| ASBT | apical sodium-dependent bile acid transporter |
| CE | cinnamon extract |
| CrA | Cranberry anthocyanin extract |
| CK | creatine kinase |
| CTGF | connective tissue growth factor |
| EGCG | epigallocatechin-3- gallate |
| eNOS | endothelial NO synthase |
| HC | high cholesterol group |
| HDL | high density lipoprotein |
| HL | HC with 20 mM luteolin at 5 mL/kg body weight group |
| HNF4α | hepatocyte nuclear factor 4α |
| HQ | HC with 20 mM quercetin at 5 mL/kg body weight group |
| LCBT | Lonicera caerulea berry polyphenol extract |
| LDH | lactate dehydrogenase |
| LDL | low-density lipoprotein |
| LDLR | low-density lipoprotein receptor |
| LXR | liver X receptor |
| MDA | malondialdehyde |
| MTP | microsomal triglyceride transfer protein |
| NC | non-cholesterol group |
| NP-C | Niemann–Pick type C |
| NPC1L1 | Niemann–Pick C1-Like 1 |
| PPARα | peroxisome proliferator activated receptor α |
| ROS | reactive oxygen species |
| RXR | retinoid X receptor |
| SREBP2 | sterol-regulatory element-binding protein 2 |
| SOD | superoxide dismutase |
| SSD | sterol-sensing domain |
| TC | total cholesterol |
| TG | triacylglycerol |
| TNF | tumor necrosis factor |
References
- Gadgil, M.D.; Anderson, C.A.; Kandula, N.R.; Kanaya, A.M. Dietary patterns in Asian Indians in the United States: An analysis of the metabolic syndrome and atherosclerosis in South Asians Living in America study. J. Acad. Nutr. Diet. 2014, 114, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Istvan, E.S.; Deisenhofer, J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science. 2001, 292, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.A. An investigative look: Selective cholesterol absorption inhibitors--embarking on a new standard of care. Am. J. Manag Care. 2002, 8, 36–39. [Google Scholar]
- van Heek, M.; Compton, D.S.; Davis, H.R. The cholesterol absorption inhibitor, ezetimibe, decreases diet-induced hypercholesterolemia in monkeys. Eur J. Pharmacol. 2001, 415, 79–84. [Google Scholar] [CrossRef]
- Altmann, S.W.; Davis, H.R. Jr.; Zhu, L.J.; Yao, X.; Hoos, L.M.; Tetzloff, G.; Iyer, S.P.; Maguire, M.; Golovko, A.; Zeng, M.; et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004, 303, 1201–1204. [Google Scholar] [CrossRef] [PubMed]
- Betters, J.L.; Yu, L. NPC1L1 and cholesterol transport. Febs Lett. 2010, 584, 2740–2747. [Google Scholar] [CrossRef] [Green Version]
- Davies, J.P.; Levy, B.; Ioannou, Y.A. Evidence for a Niemann-pick C (NPC) gene family: Identification and characterization of NPC1L1. Genomics 2000, 65, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Betters, J.L.; Yu, L. Niemann-pick C1-like 1 (NPC1L1) protein in intestinal and hepatic cholesterol transport. Annu. Rev. Physiol. 2011, 73, 239–259. [Google Scholar] [CrossRef]
- Zhang, J.H.; Ge, L.; Qi, W.; Zhang, L.; Miao, H.H.; Li, B.L.; Yang, M.; Song, B.L. The N-terminal domain of NPC1L1 protein binds cholesterol and plays essential roles in cholesterol uptake. J. Biol Chem. 2011, 286, 25088–25097. [Google Scholar] [CrossRef]
- Li, P.S.; Fu, Z.Y.; Zhang, Y.Y.; Zhang, J.H.; Xu, C.Q.; Ma, Y.T.; Li, B.L.; Song, B.L. The clathrin adaptor Numb regulates intestinal cholesterol absorption through dynamic interaction with NPC1L1. Nat. Med. 2014, 20, 80–86. [Google Scholar] [CrossRef]
- Ge, L.; Qi, W.; Wang, L.J.; Miao, H.H.; Qu, Y.X.; Li, B.L.; Song, B.L. Flotillins play an essential role in Niemann-Pick C1-like 1-mediated cholesterol uptake. Proc. Natl. Acad. Sci. USA. 2011, 108, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Wang, J.; Qi, W.; Miao, H.H.; Cao, J.; Qu, Y.X.; Li, B.L.; Song, B.L. The cholesterol absorption inhibitor ezetimibe acts by blocking the sterol-induced internalization of NPC1L1. Cell Metab. 2008, 7, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Weinglass, A.B.; Kohler, M.; Schulte, U.; Liu, J.; Nketiah, E.O.; Thomas, A.; Schmalhofer, W.; Williams, B.; Bildl, W.; McMasters, D.R.; et al. Extracellular loop C of NPC1L1 is important for binding to ezetimibe. Proc. Natl. Acad. Sci. USA 2008, 115, 11140–11145. [Google Scholar] [CrossRef] [PubMed]
- Nekohashi, M.; Ogawa, M.; Ogihara, T.; Nakazawa, K.; Kato, H.; Misaka, T.; Abe, K.; Kobayashi, S. Luteolin and quercetin affect the cholesterol absorption mediated by epithelial cholesterol transporter niemann-pick c1-like 1 in caco-2 cells and rats. PLoS ONE 2014, 23, e97901. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, K.; Tanigawara, Y.; Nakagawa, T.; Uno, T. A pharmacokinetic analysis program (multi) for microcomputer. J. Pharmacobiodyn. 1981, 4, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Yamanashi, Y.; Takada, T.; Abe, K.; Kobayashi, S. Effect of luteolin on the expression of intestinal cholesterol transporters. J Funct Foods. 2017, 36, 274–279. [Google Scholar] [CrossRef]
- Wong, T.Y.; Tan, Y.Q.; Lin, S.M.; Leung, L.K. Apigenin and luteolin display differential hypocholesterolemic mechanisms in mice fed a high-fat diet. Biomed. Pharmacother. 2017, 96, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Ohlsson, L.; Duan, R.D. Curcumin inhibits cholesterol uptake in Caco-2 cells by down-regulation of NPC1L1 expression. Lipids Health Dis. 2010, 9, 40. [Google Scholar] [CrossRef]
- Liu, S.; You, L.; Zhao, Y.; Chang, X. Wild Lonicera caerulea berry polyphenol extract reduces cholesterol accumulation and enhances antioxidant capacity in vitro and in vivo. Food Res. Int. 2018, 107, 73–83. [Google Scholar] [CrossRef]
- Feng, J.; Yang, J.; Chang, Y.; Qiao, L.; Dang, H.; Luo, K.; Guo, H.; An, Y.; Ma, C.; Shao, H.; et al. Caffeine-free hawk tea lowers cholesterol by reducing free cholesterol uptake and the production of very-low-density lipoprotein. Commun Biol. 2019, 2, 173. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhu, H.; Zhao, Y.; Jiao, R.; Lei, L.; Chen, J.; Wang, X.; Zhang, Z.; Huang, Y.; Wang, T.; et al. Cranberry anthocyanin as an herbal medicine lowers plasma cholesterol by increasing excretion of fecal sterols. Phytomedicine. 2018, 38, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Chen, J.; Zuo, Y.; Ma, K.Y.; Jiang, Y.; Huang, Y.; Chen, Z.Y. Blueberry anthocyanins at doses of 0.5 and 1 % lowered plasma cholesterol by increasing fecal excretion of acidic and neutral sterols in hamsters fed a cholesterol-enriched diet. Eur J. Nutr. 2013, 52, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Dawson, H.D.; Schoene, N.W.; Polansky, M.M.; Anderson, R.A. Cinnamon polyphenols regulate multiple metabolic pathways involved in insulin signaling and intestinal lipoprotein metabolism of small intestinal enterocytes. Nutrition. 2012, 28, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Opie, L.H.; Lecour, S. The red wine hypothesis: From concepts to protective signalling molecules. Eur. Heart J. 2007, 28, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Zern, T.L.; Fernandez, M.L. Cardioprotective effects of dietary polyphenols. J. Nutr. 2005, 135, 2291–2294. [Google Scholar] [CrossRef]
- Rasmussen, S.E.; Frederiksen, H.; Struntze Krogholm, K.; Poulsen, L. Dietary proanthocyanidins: Occurrence, dietary intake, bioavailability, and protection against cardiovascular disease. Mol. Nutr. Food Res. 2005, 49, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, I.; Yamahira, T.; Kato, M.; Ishikawa, A. Black-tea polyphenols decrease micellar solubility of cholesterol in vitro and intestinal absorption of cholesterol in rats. J. Agric. Food Chem. 2010, 58, 8591–8595. [Google Scholar] [CrossRef]
- Ikeda, I.; Imasato, Y.; Sasaki, E.; Nakayama, M.; Nagao, H.; Takeo, T.; Yayabe, F.; Sugano, M. Tea catechins decrease micellar solubility and intestinal absorption of cholesterol in rats. Biochim Biophys Acta. 1992, 1127, 141–146. [Google Scholar] [CrossRef]
- Davis, H.R. Jr.; Altmann, S.W. Niemann-Pick C1 Like 1 (NPC1L1) an intestinal sterol transporter. Biochim. Biophys. Acta. 2009, 1791, 679–683. [Google Scholar] [CrossRef]
- Annaba, F.; Kumar, P.; Dudeja, A.K.; Saksena, S.; Gill, R.K.; Alrefai, W.A. Green tea catechin EGCG inhibits ileal apical sodium bile acid transporter ASBT. Am. J. Physiol Gastrointest Liver Physiol. 2010, 298, 467–473. [Google Scholar] [CrossRef]
- Xia, X.; Jung, D.; Webb, P.; Zhang, A.; Zhang, B.; Li, L.; Ayers, S.D.; Gabbi, C.; Ueno, Y.; Gustafsson, J.Å.; et al. Liver X receptor β and peroxisome proliferator-activated receptor δ regulate cholesterol transport in murine cholangiocytes. Hepatology 2012, 56, 2288–2296. [Google Scholar] [CrossRef] [PubMed]
- Iwayanagi, Y.; Takada, T.; Suzuki, H. HNF4alpha is a crucial modulator of the cholesterol-dependent regulation of NPC1L1. Pharm Res. 2008, 25, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Iwayanagi, Y.; Takada, T.; Tomura, F.; Yamanashi, Y.; Terada, T.; Inui, K.; Suzuki, H. Human NPC1L1 expression is positively regulated by PPARα. Pharm Res. 2011, 28, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Francisco, V.; Figueirinha, A.; Costa, G.; Liberal, J.; Ferreira, I.; Lopes, M.C.; García-Rodríguez, C.; Cruz, M.T.; Batista, M.T. The Flavone Luteolin Inhibits Liver X Receptor Activation. J. Nat. Prod 2016, 79, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Lin, S.M.; Leung, L.K. The Flavone Luteolin Suppresses SREBP-2 Expression and Post-Translational Activation in Hepatic Cells. PLoS ONE 2015, 10, e0135637. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Inoue, J.; Choi, J.M.; Nakamura, S.; Yan, Z.; Fushinobu, S.; Kamada, H.; Kato, H.; Hashidume, T.; Shimizu, M.; et al. Identification of the flavonoid luteolin as a repressor of the transcription factor hepatocyte nuclear factor 4α. J. Biol. Chem. 2015, 290, 24021–24035. [Google Scholar] [CrossRef]
- Harris, D.M.; Li, L.; Chen, M.; Lagunero, F.T.; Go, V.L.; Boros, L.G. Diverse mechanisms of growth inhibition by luteolin, resveratrol, and quercetin in MIA PaCa-2 cells: A comparative glucose tracer study with the fatty acid synthase inhibitor C75. Metabolomics 2012, 8, 201–210. [Google Scholar] [CrossRef]
- Gentile, D.; Fornai, M.; Pellegrini, C.; Colucci, R.; Benvenuti, L.; Duranti, E.; Masi, S.; Carpi, S.; Nieri, P.; Nericcio, A.; et al. Luteolin prevents cardiometabolic alterations and vascular dysfunction in mice with hfd-induced obesity. Front. Pharmacol. 2018, 9, 1094. [Google Scholar] [CrossRef]
- Li, J.; Dong, J.Z.; Ren, Y.L.; Zhu, J.J.; Cao, J.N.; Zhang, J.; Pan, L.L. Luteolin decreases atherosclerosis in LDL receptor-deficient mice via a mechanism including decreasing AMPK-SIRT1 signaling in macrophages. Exp. Ther. Med. 2018, 16, 2593–2599. [Google Scholar] [CrossRef]
- Yang, J.T.; Wang, J.; Zhou, X.R.; Xiao, C.; Lou, Y.Y.; Tang, L.H.; Zhang, F.J.; Qian, L.B. Luteolin alleviates cardiac ischemia/reperfusion injury in the hypercholesterolemic rat via activating Akt/Nrf2 signaling. Naunyn Schmiedebergs Arch. Pharmacol. 2018, 391, 719–728. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Y.; Liu, C.; Xu, D.; Zhang, R.; Cheng, Y.; Pan, Y.; Huang, C.; Chen, Y. Luteolin alleviates alcoholic liver disease induced by chronic and binge ethanol feeding in mice. J. Nutr. 2014, 144, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- El-Bassossy, H.M.; Abo-Warda, S.M.; Fahmy, A. Chrysin and luteolin attenuate diabetes-induced impairment in endothelial-dependent relaxation: Effect on lipid profile, AGEs and NO generation. Phytother Res. 2013, 27, 1678–1684. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, W.; Lu, X.; Bao, P.; Zhao, X. Luteolin ameliorates cardiac failure in type I diabetic cardiomyopathy. J. Diabetes Complications. 2012, 26, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.F.; Camsari, C.; Sá, C.M.; Lima, C.F.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Ursolic acid and luteolin-7-glucoside improve lipid profiles and increase liver glycogen content through glycogen synthase kinase-3. Phytother. Res. 2010, 2, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Blazing, M.A.; Giugliano, R.P.; McCagg, A.; White, J.A.; Theroux, P.; Darius, H.; Lewis, B.S.; Ophuis, T.O.; Jukema, J.W.; et al. IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N. Engl. J. Med. 2015, 372, 2387–2397. [Google Scholar] [CrossRef] [PubMed]
- Narushima, K.; Takada, T.; Yamanashi, Y.; Suzuki, H. Niemann-pick C1-like 1 mediates alpha-tocopherol transport. Mol. Pharmacol. 2008, 74, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Knopp, R.H.; Gitter, H.; Truitt, T.; Bays, H.; Manion, C.V.; Lipka, L.J.; LeBeaut, A.P.; Suresh, R.; Yang, B.; Veltri, E.P.; et al. Effects of ezetimibe, a new cholesterol absorption inhibitor, on plasma lipids in patients with primary hypercholesterolemia. Eur. Heart J. 2003, 24, 729–741. [Google Scholar] [CrossRef] [Green Version]
- Takada, T.; Yamanashi, Y.; Konishi, K.; Yamamoto, T.; Toyoda, Y.; Masuo, Y.; Yamamoto, H.; Suzuki, H. NPC1L1 is a key regulator of intestinal vitamin K absorption and a modulator of warfarin therapy. Sci. Transl. Med. 2015, 275, 275ra23. [Google Scholar] [CrossRef]
- Alexander, B.; Goldstein, R.; Landwehr, G.; Cook, C.D. Congenital SPCA deficiency: A hitherto unrecognized coagulation defect with hemorrhage rectified by serum and serum fractions. J. Clin. Invest. 1951, 30, 596–608. [Google Scholar] [CrossRef]
- Hougie, C.; Barrow, E.M.; Graham, J.B. Stuart clotting defect. I. Segregation of an hereditary hemorrhagic state from the heterogeneous group heretofore called stable factor (SPCA, proconvertin, factor VII) deficiency. J. Clin. Invest. 1957, 36, 485–496. [Google Scholar] [CrossRef]
- Paththinige, C.S.; Sirisena, N.D.; Dissanayake, V. Genetic determinants of inherited susceptibility to hypercholesterolemia - a comprehensive literature review. Lipids Health Dis. 2017, 16, 103. [Google Scholar] [CrossRef] [PubMed]



| Flavonoids and Flavonoid-Rich Extracts | Model | Results | Reference |
|---|---|---|---|
| Luteolin, quercetin | Caco-2, Wistar rat (male) | Pretreated the Caco-2 cells with 100 µM luteolin for 1 h to inhibit cholesterol uptake. Luteolin (20 mM) and quercetin at 5 mL/kg body weight diet decreased the cholesterol concentration in rat. | [14] |
| Luteolin | Caco-2 | The 100 µM luteolin-treated Caco-2 cells significantly attenuated the NPC1L1 mRNA and protein levels. | [16] |
| Luteolin | C57BL / 6 mouse (male) | After 8 weeks of treatment, the administration of 250 ppm luteolin could modulate the total and serum non-HDL cholesterol. Npc1l1 mRNA expressions were decreased in the intestinal mucosa of luteolin-fed animals. | [17] |
| Curucumin | Caco-2 | Pretreated the Caco-2 cells with 25–100µM curcumin for 24 h showing a dose-dependent inhibition of cholesterol uptake. Curcumin decreased the levels of the NPC1L1 protein | [18] |
| Wild Lonicera caerulea berry polyphenol extract (LCBT), cyanidin-3-glucoside, catechin, chlorogenic acid | Caco-2, Wistar rat (male) | Treatment the Caco-2 cells with LCBT and 50 µM cyanidin-3-glucoside, catechin, and chlorogenic acid for 24 h down-regulated NPC1L1 mRNA expression. LCBP supplementation at 150 and 300mg/kg decreased the TC, TG, and LDL-C levels but increased the HDL-C level. Intestinal NPC1L1 protein expression was reduced in the LCBP treatment group. | [19] |
| Caffeine-free hawk tea, EGCG | HepG2, Caco2, CRL1601/NPC1L1-EGFP, plasma membrane Sprague–Dawley rat (male) | Hawk tea extract inhibited NPC1L1-mediated free cholesterol uptake, which induced the transcription of low-density lipoprotein receptor downstream the sterol response element binding protein 2 pathway in hepatocytes. EGCG inhibits the endocytosis of NPC1L1 from the plasma membrane to endocytic recycling compartment. | [20] |
| Cranberry anthocyanin extract (CrA) | Hamster | Plasma total cholesterol and aorta atherosclerotic plaque decreased in a dose-dependent manner with increasing amounts of 1% and 2% CrA added into diets. CrA had no effect on the mRNA levels of intestinal NPC1L1. | [21] |
| Blueberry anthocyanins extract | Hamster | Dietary supplementation of 0.5% and 1.0% blueberry anthocyanins for 6 weeks decreased plasma TC concentration and increased the excretion of fecal neutral and acidic sterols. Blueberry anthocyanins down-regulated the gene expression of NPC1L1. | [22] |
| Aqueous cinnamon extract (CE) | Wistar rat (male) Primary enterocytes | The intestinal Npc1l1 mRNA levels were lower after treatment for 2 h of 10 μg/mL CE treatment and for 4 h of 100 μg/mL cinnamon extract treatment. | [23] |
| Model | Anti-Hypercholesterolemia Effects | Reference |
|---|---|---|
| HepG2, RAW 264.7 | Luteolin abrogated the LXRα/β transcriptional activity and, subsequently, inhibited SREBP-1c expression, lipid accumulation, and ABCA1 expression. | [34] |
| HepG2, non-cancer WRL | Luteolin suppressed the expression, nuclear translocation, and transcription of SREBP-2 in the hepatic cell lines. The transcription of HMGCR also decreased after luteolin treatment. | [35] |
| HepG2, Caco-2, C57BL/6 mouse (male) | The activity of the MTP gene promoter was suppressed by luteolin. Luteolin decreased the mRNA levels of HNF4α target genes and inhibited apoB-containing lipoprotein secretion. Luteolin suppressed the acetylation level of histone H3 in the promoter region of certain HNF4α target genes. Luteolin treatment of mice for 57 days lowered serum VLDL and LDL cholesterol, and apoB protein levels without accumulating fat in the liver. | [36] |
| MIA PaCa-2 | Luteolin inhibited fatty acid synthesis from acetyl-CoA by blocking fatty acid synthase. | [37] |
| C57BL/6 mouse(male) | In HFD mice, luteolin counteracted the increase in body, epididymal fat weight, and the associated metabolic alterations. Luteolin restored vascular endothelial NO availability, normalized the media–lumen ratio, decreased ROS and TNF levels, and normalized eNOS, SOD1 and microRNA-214-3p expression. | [38] |
| C57BL/6 mouse (male) | Luteolin prevents plaque development and lipid accumulation in the abdominal aorta by decreasing macrophage inflammation during atherosclerosis, which is mediated by mechanisms including AMPK-SIRT1 signaling. | [39] |
| Sprague–Dawley rat(male) | Luteolin protects the hypercholesterolemic heart against ischemia/reperfusion injury due to upregulation of Akt-mediated Nrf2 antioxidative function and inhibition of mitochondrial permeability transition pore. | [40] |
| C57BL/6 mouse (male), AML-12, L-02 | Compared with the EtOH group, the EtOH + luteolin group had reductions in serum ALT, TG, LDL cholesterol, and lipid accumulation in the liver. Luteolin reduced ethanol-induced expression of Srebp1c, Fasn, Acc, and Scd1 genes in the liver. In cultured hepatocytes, luteolin prevented alcohol-induced lipid accumulation and increased the expression of lipogenic genes. | [41] |
| Wistar rat (male) | Luteolin prevented increase in the levels of TGs, total and LDL cholesterol in a streptozotocin-injected diabetes model. | [42] |
| Sprague–Dawley rat (male) | Luteolin treatment induced a decrease in serum TG, TC, LDL, MDA, CK, LDH, and myocardial CTGF and a significant increase in HDL, SOD and Akt phosphorylation levels in comparison with a diabetics group. | [43] |
| Wistar rat (male) | Luteolin-7-glucoside was assessed in vivo in healthy rats for the effects on plasma glucose and lipid profile (total cholesterol, HDL and LDL), as well as liver glycogen content in a diabetes model. | [44] |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, S. The Effect of Polyphenols on Hypercholesterolemia through Inhibiting the Transport and Expression of Niemann–Pick C1-Like 1. Int. J. Mol. Sci. 2019, 20, 4939. https://doi.org/10.3390/ijms20194939
Kobayashi S. The Effect of Polyphenols on Hypercholesterolemia through Inhibiting the Transport and Expression of Niemann–Pick C1-Like 1. International Journal of Molecular Sciences. 2019; 20(19):4939. https://doi.org/10.3390/ijms20194939
Chicago/Turabian StyleKobayashi, Shoko. 2019. "The Effect of Polyphenols on Hypercholesterolemia through Inhibiting the Transport and Expression of Niemann–Pick C1-Like 1" International Journal of Molecular Sciences 20, no. 19: 4939. https://doi.org/10.3390/ijms20194939