Inhibitory Effects of a Novel Chrysin-Derivative, CPD 6, on Acute and Chronic Skin Inflammation
Abstract
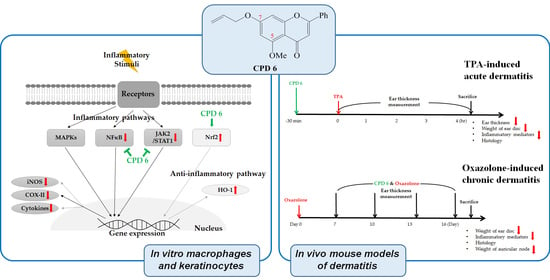
:1. Introduction
2. Results
2.1. Flavonoid and Its Derivatives Inhibit the Release of NO in LPS-Activated Murine Macrophages
2.2. Inhibitory Effect of CPD 6 on the Release of Inflammatory Mediators from LPS-Stimulated Macrophages
2.3. CPD 6 Downregulates Inflammatory Mediators in Keratinocytes Stimulated by TNF-α/IFN-γ, UV, or Chemical Irritant
2.4. Suppression of Acute Skin Inflammation Induced by Phorbol 12-Myristate 13-Acetate (TPA) by CPD 6 in Mice
2.5. Protective Effect of CPD 6 on Chronic Skin Inflammation Induced by Oxazolone in Mice
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of CPD 1 to 14
4.3. Cell Culture
4.4. UV Irradiation
4.5. Determination of Cell Viability
4.6. Determination of the Released Levels of NO and PGE2
4.7. Mesurement of Protein Levels in Western Blot
4.8. Determination of mRNA Levels in Quantitative Real Time PCR (qRT-PCR)
4.9. Animals
4.10. Mouse Models of Acute Ear Inflammation Induced by TPA
4.11. Mouse Models of Chronic Dermatitis Induced by Oxazolone
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| COX-II | Cyclooxygenase II |
| CPD 6 | Compound 6 |
| Dexa | Dexamethasone |
| DMSO | Dimethyl sulfoxide |
| ERK | Extracellular signal-regulated kinase |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| H&E | Hematoxylin and eosin |
| INF-γ | Interferon-gamma |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| INDO | Indomethacin |
| iNOS | Inducible nitric oxide synthase |
| JAK | Janus kinase |
| JNK | c-Jun N-terminal kinase |
| L-NAME | N-nitro-L-arginine methyl ester hydrochloride |
| LPS | Lipopolysaccharide |
| MAPKs | Mitogen activated protein kinase |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NFκB | Nuclear factor kappa B |
| NO | Nitric oxide |
| Nrf2 | Nuclear factor-erythroid 2-related factor-2 |
| Oxaz | Oxazolone (4-ethoxymethylene-2-phenyl-2-oxazolin-5-one) |
| PGE2 | Prostaglandin E2 |
| STAT | Signal Transducers and Activators of Transcription |
| TB | Toluidine blue |
| TNF-α | Tumor necrosis factor-alpha |
| TPA | Phorbol 12-myristate 13-acetate |
References
- Barker, J.N.; Mitra, R.S.; Griffiths, C.E.; Dixit, V.M.; Nickoloff, B.J. Keratinocytes as initiators of inflammation. Lancet 1991, 337, 211–214. [Google Scholar] [CrossRef] [Green Version]
- Carvajal-Vidal, P.; Mallandrich, M.; Garcia, M.L.; Calpena, A.C. Effect of Different Skin Penetration Promoters in Halobetasol Propionate Permeation and Retention in Human Skin. Int. J. Mol. Sci. 2017, 18, 2475. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arter. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Feghali, C.A.; Wright, T.M. Cytokines in acute and chronic inflammation. Front. Biosci. 1997, 2, d12–d26. [Google Scholar] [PubMed]
- Pasparakis, M.; Haase, I.; Nestle, F.O. Mechanisms regulating skin immunity and inflammation. Nat. Rev. Immunol. 2014, 14, 289–301. [Google Scholar] [CrossRef]
- Kang, M.C.; Cho, K.; Lee, J.H.; Subedi, L.; Yumnam, S.; Kim, S.Y. Effect of Resveratrol-Enriched Rice on Skin Inflammation and Pruritus in the NC/Nga Mouse Model of Atopic Dermatitis. Int. J. Mol. Sci. 2019, 20, 1428. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Matsubara, M.; Takada, C.; Hasegawa, K.; Suzuki, K.; Ohmori, K.; Karasawa, A. Effects of olopatadine hydrochloride, an antihistamine drug, on skin inflammation induced by repeated topical application of oxazolone in mice. Br. J. Dermatol. 2004, 151, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Eder, W.; Ege, M.J.; von Mutius, E. The asthma epidemic. N. Engl. J. Med. 2006, 355, 2226–2235. [Google Scholar] [CrossRef]
- Thomsen, S.F. Atopic dermatitis: natural history, diagnosis, and treatment. ISRN Allergy 2014, 2014, 354250. [Google Scholar] [CrossRef]
- Siegfried, E.C.; Jaworski, J.C.; Kaiser, J.D.; Hebert, A.A. Systematic review of published trials: long-term safety of topical corticosteroids and topical calcineurin inhibitors in pediatric patients with atopic dermatitis. BMC Pediatr 2016, 16, 75. [Google Scholar] [CrossRef] [PubMed]
- Chae, B.S. Pretreatment of Low-Dose and Super-Low-Dose LPS on the Production of In Vitro LPS-Induced Inflammatory Mediators. Toxicol. Res. 2018, 34, 65–73. [Google Scholar] [CrossRef]
- Bae, O.N.; Noh, M.; Chun, Y.J.; Jeong, T.C. Keratinocytic vascular endothelial growth factor as a novel biomarker for pathological skin condition. Biomol. Ther. 2015, 23, 12–18. [Google Scholar] [CrossRef]
- Kundu, J.; Kim, D.H.; Chae, I.G.; Lee, J.K.; Lee, S.; Jeong, C.H.; Chun, K.S. Silicon dioxide nanoparticles induce COX-2 expression through activation of STAT3 signaling pathway in HaCaT cells. Toxicol. In Vitro 2018, 52, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Beyer, T.A.; Auf dem Keller, U.; Braun, S.; Schafer, M.; Werner, S. Roles and mechanisms of action of the Nrf2 transcription factor in skin morphogenesis, wound repair and skin cancer. Cell Death Differ. 2007, 14, 1250–1254. [Google Scholar] [CrossRef] [PubMed]
- Pae, H.O.; Chung, H.T. Heme oxygenase-1: its therapeutic roles in inflammatory diseases. Immune. Netw. 2009, 9, 12–19. [Google Scholar] [CrossRef]
- Doi, M.; Shichiri, M.; Katsuyama, K.; Ishimaru, S.; Hirata, Y. Cytokine-activated Jak-2 is involved in inducible nitric oxide synthase expression independent from NF-kappaB activation in vascular smooth muscle cells. Atherosclerosis 2002, 160, 123–132. [Google Scholar] [CrossRef]
- Ganster, R.W.; Taylor, B.S.; Shao, L.; Geller, D.A. Complex regulation of human inducible nitric oxide synthase gene transcription by Stat 1 and NF-kappa B. Proc. Natl. Acad. Sci. USA 2001, 98, 8638–8643. [Google Scholar] [CrossRef]
- Liu, P.W.; Chen, M.F.; Tsai, A.P.; Lee, T.J. STAT1 mediates oroxylin a inhibition of iNOS and pro-inflammatory cytokines expression in microglial BV-2 cells. PLoS ONE 2012, 7, e50363. [Google Scholar] [CrossRef] [PubMed]
- Tsoyi, K.; Kim, H.J.; Shin, J.S.; Kim, D.H.; Cho, H.J.; Lee, S.S.; Ahn, S.K.; Yun-Choi, H.S.; Lee, J.H.; Seo, H.G.; et al. HO-1 and JAK-2/STAT-1 signals are involved in preferential inhibition of iNOS over COX-2 gene expression by newly synthesized tetrahydroisoquinoline alkaloid, CKD712, in cells activated with lipopolysacchride. Cell Signal 2008, 20, 1839–1847. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Shin, I.; Kim, K.A.; Noh, D.; Baek, S.H.; Chang, S.Y.; Kim, H.; Bae, O.N. A newly synthesized macakurzin C-derivative attenuates acute and chronic skin inflammation: The Nrf2/heme oxygenase signaling as a potential target. Toxicol. Appl. Pharmacol. 2016, 307, 62–71. [Google Scholar] [CrossRef]
- Lim, H.; Heo, M.Y.; Kim, H.P. Flavonoids: Broad Spectrum Agents on Chronic Inflammation. Biomol. Ther. 2019, 27, 241–253. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Choi, J.K.; Jang, Y.H.; Lee, S.; Lee, S.R.; Choi, Y.A.; Jin, M.; Choi, J.H.; Park, J.H.; Park, P.H.; Choi, H.; et al. Chrysin attenuates atopic dermatitis by suppressing inflammation of keratinocytes. Food Chem. Toxicol. 2017, 110, 142–150. [Google Scholar] [CrossRef]
- Akram, M.; Syed, A.S.; Kim, K.A.; Lee, J.S.; Chang, S.Y.; Kim, C.Y.; Bae, O.N. Heme oxygenase 1-mediated novel anti-inflammatory activities of Salvia plebeia and its active components. J. Ethnopharmacol. 2015, 174, 322–330. [Google Scholar] [CrossRef]
- Gilmore, T.D. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene 2006, 25, 6680–6684. [Google Scholar] [CrossRef]
- Liu, Y.; Shepherd, E.G.; Nelin, L.D. MAPK phosphatases--regulating the immune response. Nat. Rev. Immunol. 2007, 7, 202–212. [Google Scholar] [CrossRef]
- Akram, M.; Kim, K.A.; Kim, E.S.; Shin, Y.J.; Noh, D.; Kim, E.; Kim, J.H.; Majid, A.; Chang, S.Y.; Kim, J.K.; et al. Selective inhibition of JAK2/STAT1 signaling and iNOS expression mediates the anti-inflammatory effects of coniferyl aldehyde. Chem. Biol. Interact. 2016, 256, 102–110. [Google Scholar] [CrossRef]
- Jacobs, A.T.; Ignarro, L.J. Lipopolysaccharide-induced expression of interferon-beta mediates the timing of inducible nitric-oxide synthase induction in RAW 264.7 macrophages. J. Biol. Chem. 2001, 276, 47950–47957. [Google Scholar] [CrossRef]
- Dunaway, S.; Odin, R.; Zhou, L.; Ji, L.; Zhang, Y.; Kadekaro, A.L. Natural Antioxidants: Multiple Mechanisms to Protect Skin From Solar Radiation. Front. Pharmacol. 2018, 9, 392. [Google Scholar] [CrossRef] [Green Version]
- Murphy, J.E.; Robert, C.; Kupper, T.S. Interleukin-1 and cutaneous inflammation: a crucial link between innate and acquired immunity. J. Invest. Dermatol. 2000, 114, 602–608. [Google Scholar] [CrossRef]
- Mani, R.; Natesan, V. Chrysin: Sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry 2018, 145, 187–196. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.P.; He, J.; Liu, D.; Zhang, Q.Z.; Li, K.; Zheng, X.; Tang, G.T.; Guo, Y.; Liu, Y. The Relationship between Pharmacological Properties and Structure- Activity of Chrysin Derivatives. Mini. Rev. Med. Chem. 2019, 19, 555–568. [Google Scholar] [CrossRef]
- Song, H.Y.; Kim, W.S.; Mushtaq, S.; Park, J.M.; Choi, S.H.; Cho, J.W.; Lim, S.T.; Byun, E.B. A novel chrysin derivative produced by gamma irradiation attenuates 2,4-dinitrochlorobenzene-induced atopic dermatitis-like skin lesions in Balb/c mice. Food Chem. Toxicol. 2019, 128, 223–232. [Google Scholar] [CrossRef]
- Deckers, I.A.; McLean, S.; Linssen, S.; Mommers, M.; van Schayck, C.P.; Sheikh, A. Investigating international time trends in the incidence and prevalence of atopic eczema 1990-2010: a systematic review of epidemiological studies. PLoS ONE 2012, 7, e39803. [Google Scholar] [CrossRef]
- Kou, X.; Qi, S.; Dai, W.; Luo, L.; Yin, Z. Arctigenin inhibits lipopolysaccharide-induced iNOS expression in RAW264.7 cells through suppressing JAK-STAT signal pathway. Int. Immunopharmacol. 2011, 11, 1095–1102. [Google Scholar] [CrossRef]
- Miklossy, G.; Hilliard, T.S.; Turkson, J. Therapeutic modulators of STAT signalling for human diseases. Nat. Rev. Drug Discov. 2013, 12, 611–629. [Google Scholar] [CrossRef] [Green Version]
- Kang, N.J.; Han, S.C.; Kang, H.J.; Ko, G.; Yoon, W.J.; Kang, H.K.; Yoo, E.S. Anti-Inflammatory Effect of 3-Bromo-4,5-Dihydroxybenzaldehyde, a Component of Polysiphonia morrowii, In Vivo and In Vitro. Toxicol. Res. 2017, 33, 325–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative Stress, Inflammation, and Cancer: How are They Linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Shi, Y.; Tu, Z.; Tang, D.; Zhang, H.; Liu, M.; Wang, K.; Calderwood, S.K.; Xiao, X. The inhibition of LPS-induced production of inflammatory cytokines by HSP70 involves inactivation of the NF-kappaB pathway but not the MAPK pathways. Shock 2006, 26, 277–284. [Google Scholar] [CrossRef]
- Shin, Y.W.; Bae, E.A.; Kim, S.S.; Lee, Y.C.; Kim, D.H. Effect of ginsenoside Rb1 and compound K in chronic oxazolone-induced mouse dermatitis. Int. Immunopharmacol. 2005, 5, 1183–1191. [Google Scholar] [CrossRef]
- Abraham, N.G.; Kappas, A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol. Rev. 2008, 60, 79–127. [Google Scholar] [CrossRef] [PubMed]
- Listopad, J.; Asadullah, K.; Sievers, C.; Ritter, T.; Meisel, C.; Sabat, R.; Docke, W.D. Heme oxygenase-1 inhibits T cell-dependent skin inflammation and differentiation and function of antigen-presenting cells. Exp. Dermatol. 2007, 16, 661–670. [Google Scholar] [CrossRef]
- Ndisang, J.F. Role of heme oxygenase in inflammation, insulin-signalling, diabetes and obesity. Mediat. Inflamm. 2010, 2010, 359732. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta. Mol. Basis. Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Lee, T.H.; Oh, H.J.; Kim, H.; Son, Y.; Lee, E.H.; Kim, J. Inhibitory effect of 5,6-dihydroergosteol-glucoside on atopic dermatitis-like skin lesions via suppression of NF-kappaB and STAT activation. J. Dermatol. Sci. 2015, 79, 252–261. [Google Scholar] [CrossRef]
- Liao, B.C.; Hsieh, C.W.; Liu, Y.C.; Tzeng, T.T.; Sun, Y.W.; Wung, B.S. Cinnamaldehyde inhibits the tumor necrosis factor-alpha-induced expression of cell adhesion molecules in endothelial cells by suppressing NF-kappaB activation: effects upon IkappaB and Nrf2. Toxicol. Appl. Pharmacol. 2008, 229, 161–171. [Google Scholar] [CrossRef]
- Song, M.Y.; Kim, E.K.; Moon, W.S.; Park, J.W.; Kim, H.J.; So, H.S.; Park, R.; Kwon, K.B.; Park, B.H. Sulforaphane protects against cytokine- and streptozotocin-induced beta-cell damage by suppressing the NF-kappaB pathway. Toxicol. Appl. Pharmacol. 2009, 235, 57–67. [Google Scholar] [CrossRef]
- Lastowiecka-Moras, E.; Bugajska, J.; Mlynarczyk, B. Occupational exposure to natural UV radiation and premature skin ageing. Int. J. Occup. Saf. Ergon. 2014, 20, 639–645. [Google Scholar] [CrossRef]
- Bull, J.E.; Parker, D.; Turk, J.L. Predictive value of assessment of lymph node weight and T-lymphocyte proliferation in contact sensitivity in acrylates. J. Invest. Dermatol. 1985, 85, 403–406. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Alysandratos, K.D.; Angelidou, A.; Delivanis, D.A.; Sismanopoulos, N.; Zhang, B.; Asadi, S.; Vasiadi, M.; Weng, Z.; Miniati, A.; et al. Mast cells and inflammation. Biochim. Biophys. Acta. 2012, 1822, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Rotsztejn, W.H.; Besson, J.; Briaud, B.; Gagnant, L.; Rosselin, G.; Kordon, C. Effect of steroids on vasoactive intestinal peptide in discrete brain regions and peripheral tissues. Neuroendocrinology 1980, 31, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Caslin, H.L.; Kiwanuka, K.N.; Haque, T.T.; Taruselli, M.T.; MacKnight, H.P.; Paranjape, A.; Ryan, J.J. Controlling Mast Cell Activation and Homeostasis: Work Influenced by Bill Paul That Continues Today. Front. Immunol. 2018, 9, 868. [Google Scholar] [CrossRef] [PubMed]
- Bäumer, W.; Krekeler, S.; DeVries, V.C.; Niedorf, F.; Tschernig, T.; Kietzmann, M. Non-steroidal and steroidal anti-inflammatory drugs vary in their modulation of dendritic cell function in the elicitation phase of allergic contact dermatitis. Exp. Dermatol. 2006, 15, 322–329. [Google Scholar] [CrossRef] [PubMed]





| Groups | Histological Score (Mean ± S.D.) | ||
|---|---|---|---|
| Ear Edema | Epidermal Hyper Proliferation | Leucocyte Infiltration | |
| Control | 0.03 ± 0.129 | 0.03 ± 0.129 | 0.00 ± 0.000 |
| TPA | 3.33 ± 0.772 | 2.13 ± 0.767 | 2.90 ± 0.507 |
| CPD 6 (0.2 mg/ear) + TPA | 1.80 ± 0.841 ** | 1.10 ± 0.632 ** | 2.30 ± 0.21 |
| CPD 6 (0.4 mg/ear) + TPA | 1.77 ± 0.942 ** | 0.67 ± 0.645 ** | 1.83 ± 1.097 ** |
| Indo (0.2 mg/ear) + TPA | 1.27 ± 1.033 ** | 0.87 ± 0.767 ** | 1.80 ± 0.841 ** |
| CPD 6 (0.4 mg/ear) | 0.17 ± 0.244 | 0.07 ± 0.176 | 0.47 ± 0.481 |
| Groups | Histological Score (mean ± S.D.) | ||
|---|---|---|---|
| Ear Edema | Epidermal Hyper Proliferation | Leucocyte Infiltration | |
| Control | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.00 ± 0.000 |
| Oxaz | 4.00 ± 0.667 | 4.13 ± 0.447 | 4.8 ± 0.183 |
| CPD 6 (0.2 mg/ear) + Oxaz | 3.07 ± 0.830 | 3.07 ± 0.683 ** | 3.60 ± 1.140 * |
| CPD 6 (0.4 mg/ear) + Oxaz | 2.47 ± 0.869 ** | 2.60 ± 0.279 ** | 3.20 ± 0.558 ** |
| Dexa (0.2 mg/ear) + Oxaz | 0.67 ± 0.471 ** | 0.93 ± 0.596 ** | 0.73 ± 0.494 ** |
| CPD 6 (0.4 mg/ear) | 0.00 ± 0.000 | 0.07 ± 0.149 | 0.00 ± 0.000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.-H.; Suh, B.; Shin, I.; Kim, E.-H.; Kim, D.; Shin, Y.-J.; Chang, S.-Y.; Baek, S.-H.; Kim, H.; Bae, O.-N. Inhibitory Effects of a Novel Chrysin-Derivative, CPD 6, on Acute and Chronic Skin Inflammation. Int. J. Mol. Sci. 2019, 20, 2607. https://doi.org/10.3390/ijms20112607
Yu C-H, Suh B, Shin I, Kim E-H, Kim D, Shin Y-J, Chang S-Y, Baek S-H, Kim H, Bae O-N. Inhibitory Effects of a Novel Chrysin-Derivative, CPD 6, on Acute and Chronic Skin Inflammation. International Journal of Molecular Sciences. 2019; 20(11):2607. https://doi.org/10.3390/ijms20112607
Chicago/Turabian StyleYu, Chan-Hee, Beomseon Suh, Iljin Shin, Eun-Hye Kim, Donghyun Kim, Young-Jun Shin, Sun-Young Chang, Seung-Hoon Baek, Hyoungsu Kim, and Ok-Nam Bae. 2019. "Inhibitory Effects of a Novel Chrysin-Derivative, CPD 6, on Acute and Chronic Skin Inflammation" International Journal of Molecular Sciences 20, no. 11: 2607. https://doi.org/10.3390/ijms20112607