The Late Stages of Melanogenesis: Exploring the Chemical Facets and the Application Opportunities
Abstract
:1. Melanin Pigmentation and Melanocytes
2. Melanogenesis with Focus on the Late Stages
3. Structure-Properties Relationship of Melanin Pigments
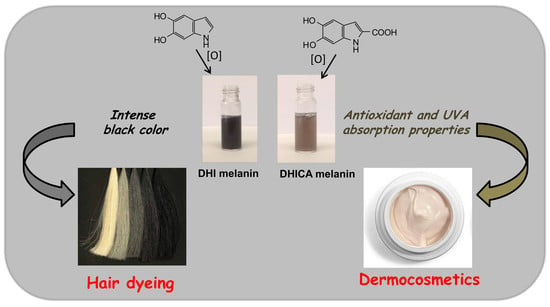
4. Exploiting the Uniqueness of Melanin Chemistry
4.1. Hair Dyeing with Melanin Precursors
4.1.1. Gray Hair and Hair Dyes
4.1.2. Melanin Precursors and Their Selection for Hair Dyes
- (1)
- Sustainable product: this dyestuff is a natural origin and sustainable material.
- (2)
- Genuine natural shade results: DHI produces original color of melanin.
- (3)
- Hydrogen peroxide-free formulation: it means no need to mix (easiness to use), and less damage on hair.
DHI Manufacturing Process
Dyeing Mechanism and Advantages of the Use of the DHI-Based Dye
4.2. Eumelanin Based Cosmetic Ingredients
5. Conclusions
6. Patents
- Kao Corporation. Production process for indoles and indolins. JP-Patent 4653333, 30 March 2001.
- Gekkeikan Sake Company; Kao Corporation. Hair dye composition. JP-Patent 4955920, 4 December 2004.
- Gekkeikan Sake Company; Kao Corporation. Hair dye composition. JP-Patent 4395436, 4 December 2004.
- Kao Corporation. Production process for indoles and indolins. JP-Patent 5507786, 7 June 2006.
- Kao Corporation. Production process for indoles and indolins. JP-Patent 5043369, 7 June 2006.
- Kao Corporation. Production process for indoles and indolins. JP-Patent 5043368, 7 June 2006.
Acknowledgments
Conflicts of Interest
Abbreviations
| 18-MEA | 18-methyl eicosanoic acid |
| Dct | dopachrome tautomerase |
| DHI | 5,6-dihydroxyindole |
| DHICA | 5,6-dihydroxyindole-2-carboxyl acid |
| HUPF | hair ultraviolet protection factor |
| MALDI MS | Matrix Assisted Laser Desorption Ionization Mass Spectrometry |
| PVA | Polyvinyl alcohol |
| Tyrp2 | tyrosinase-related protein-2 |
| UV-A | UltraViolet-A |
References
- Cichorek, M.; Wachulska, M.; Stasiewicz, A.; Tyminska, A. Skin melanocytes: Biology and development. Postepy Dermatol. Alergol. 2013, 30, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Hachiya, A.; Sriwiriyanont, P.; Kaiho, E.; Kitahara, T.; Takema, Y.; Tsuboi, R. An in vivo mouse model of human skin substitute containing spontaneously sorted melanocytes demonstrates physiological changes after UVB irradiation. J. Investig. Dermatol. 2005, 125, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.D.; Peles, D.N. The red and the black. Acc. Chem. Res. 2010, 43, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Wakamatsu, K.; d’Ischia, M.; Napolitano, A.; Pezzella, A. Structure of melanins. In Melanins and Melanosomes: Biosynthesis, Biogenesis, Physiological, and Pathological Functions; Riley, P.A., Borovansky, J., Eds.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2011. [Google Scholar]
- Robbins, C.R. Chemical and Physical Behavior of Human Hair, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Liu, Y.; Hong, L.; Wakamatsu, K.; Ito, S.; Adhyaru, B.; Cheng, C.Y.; Bowers, C.R.; Simon, J.D. Comparison of structural and chemical properties of black and red human hair melanosomes. Photochem. Photobiol. 2005, 81, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kempf, V.R.; Nofsinger, J.B.; Weinert, E.E.; Rudnicki, M.; Wakamatsu, K.; Ito, S.; Simon, J.D. Comparison of the structural and physical properties of human hair eumelanin following enzymatic or acid/base extraction. Pigment Cell Res. 2003, 16, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.D.; Hong, L.; Peles, D.N. Insights into melanosomes and melanin from some interesting spatial and temporal properties. J. Phys. Chem. B 2008, 112, 11201–11217. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Wakamatsu, K. Quantitative analysis of eumelanin and pheomelanin in humans, mice, and other animals. Pigment Cell Res. 2003, 16, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Wakamatsu, K. Human hair melanins: What we have learned and have not learned from mouse coat color pigmentation. Pigment Cell Melanoma Res. 2011, 24, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Miyake, S.; Maruyama, S.; Suzuki, I.; Commo, S.; Nakanishi, Y.; Wakamatsu, K. Acid hydrolysis reveals a low but constant level of pheomelanin in human black to brown hair. Pigment Cell Melanoma Res. 2018, 31, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Koike, K.; Yoshida, O.; Mamada, A.; Watanabe, T.; Hasegawa, T.; Kuwae, A.; Takaoka, A. Structural analysis of human hair fibers under the ultra-high voltage electron microscope. J. Cosmet. Sci. 2004, 55, S25–S27. [Google Scholar] [CrossRef] [PubMed]
- Durairaj, C.; Chastain, J.E.; Kompella, U.B. Intraocular distribution of melanin in human, monkey, rabbit, minipig and dog eyes. Exp. Eye Res. 2012, 98, 23–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef]
- Watabe, H.; Kushimoto, T.; Valencia, J.C.; Hearing, V.J. Isolation of melanosomes. Curr. Protoc. Cell Biol. 2005, 3, 3–14. [Google Scholar]
- Tadokoro, R.; Takahashi, Y. Intercellular transfer of organelles during body pigmentation. Curr. Opin. Genet. Dev. 2017, 45, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Akazaki, S.; Takahashi, T.; Nakano, Y.; Nishida, T.; Mori, H.; Takaoka, A.; Aoki, H.; Chen, H.; Kunisada, T.; Koike, K. Three-dimensional analysis of melanosomes isolated from B16 melanoma cells by using ultra high voltage electron microscopy. Microsc. Res. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- Gorniak, T.; Haraszti, T.; Garamus, V.M.; Buck, A.R.; Senkbeil, T.; Priebe, M.; Hedberg-Buenz, A.; Koehn, D.; Salditt, T.; Grunze, M.; et al. Nano-scale morphology of melanosomes revealed by small-angle X-ray scattering. PLoS ONE 2014, 9, e90884. [Google Scholar] [CrossRef] [PubMed]
- Raper, H.S. The tyrosinase-tyrosine reaction. VI. Production from tyrosine of 5,6-dihydroxyindole and 5,6-dihydroxyindole-2-carboxylic acid—The precursors of melanin. Biochem. J. 1927, 21, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Mason, H.S. The chemistry of melanin. III. Mechanism of the oxidation of dihydroxyphenylalanine by tyrosinase. J. Biol. Chem. 1948, 172, 83–99. [Google Scholar] [PubMed]
- Ito, S.; Wakamatsu, K. Chemistry of mixed melanogenesis—Pivotal roles of dopaquinone. Photochem. Photobiol. 2008, 84, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Prota, G. A facile one-step synthesis of cysteinyldopas using mushroom tyrosinase. Experientia 1977, 33, 1118–1119. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, A.; Panzella, L.; Leone, L.; d’Ischia, M. Red hair benzothiazines and benzothiazoles: Mutation-inspired chemistry in the quest for functionality. Acc. Chem. Res. 2013, 46, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Micillo, R.; Panzella, L.; Koike, K.; Monfrecola, G.; Napolitano, A.; d’Ischia, M. “Fifty shades” of black and red or how carboxyl groups fine tune eumelanin and pheomelanin properties. Int. J. Mol. Sci. 2016, 17, 746. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Napolitano, A.; d’Ischia, M. Is DHICA the key to dopachrome tautomerase and melanocyte functions? Pigment Cell Melanoma Res. 2011, 24, 248–249. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.K.; Hearing, V.J.; Urabe, K.; Aroca, P.; Spritz, R.A. Mutational mapping of the catalytic activities of human tyrosinase. J. Biol. Chem. 1991, 267, 23707–23712. [Google Scholar]
- Edge, R.; d’Ischia, M.; Land, E.J.; Napolitano, A.; Navarantnam, S.; Panzella, L.; Pezzella, A.; Ramsden, C.A.; Riley, P.A. Dopaquinone redox exchange with dihydroxyindole and dihydroxyindole-2-carboxylic acid. Pigment Cell Res. 2006, 19, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Cervantes, C.; Solano, F.; Kobayashi, T.; Urabe, K.; Hearing, V.J.; Lozano, J.A.; Garcia-Borron, J.C. A new enzymatic function in the melanogenic pathway. The 5,6-dihydroxyindole-2-carboxylic acid oxidase activity of tyrosinaserelated protein-1 (TRP1). J. Biol. Chem. 1994, 269, 17993–18001. [Google Scholar] [PubMed]
- Kobayashi, T.; Urabe, K.; Winder, A.J.; Jimenez-Cervantes, C.; Imokawa, G.; Brewington, T.; Solano, F.; Garcia-Borron, J.C.; Hearing, V.J. Tyrosinase related protein 1 (TRP1) functions as a DHICA oxidase in melanin biosynthesis. EMBO J. 1994, 13, 5818–5825. [Google Scholar] [PubMed]
- Boissy, R.E.; Sakai, C.; Zhao, H.; Kobayashi, T.; Hearing, V.J. Human tyrosinase-related protein-1 (TRP-1) does not function as a DHICA oxidase in contrast to murine TRP-1. Exp. Dermatol. 1998, 7, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. On the metal cofactor in the tyrosinase family. Int. J. Mol. Sci. 2018, 19, 633. [Google Scholar] [CrossRef] [PubMed]
- D’Ischia, M.; Wakamatsu, K.; Napolitano, A.; Briganti, S.; Garcia-Borron, J.C.; Kovacs, D.; Meredith, P.; Pezzella, A.; Picardo, M.; Sarna, T.; et al. Melanins and melanogenesis: Methods, standards, protocols. Pigment Cell Melanoma Res. 2013, 26, 616–633. [Google Scholar] [CrossRef] [PubMed]
- D’Ischia, M.; Napolitano, A.; Pezzella, A.; Land, E.J.; Ramsden, C.A.; Riley, P.A. 5,6-Dihydroxyindoles and indole-5,6-diones. Adv. Heterocycl. Chem. 2005, 89, 1–63. [Google Scholar]
- D’Ischia, M.; Napolitano, A.; Pezzella, A. 5,6-dihydroxyindole chemistry: Unexplored opportunities beyond eumelanin. Eur. J. Org. Chem. 2011, 2011, 5501–5516. [Google Scholar] [CrossRef]
- Corradini, M.G.; Napolitano, A.; Prota, G. A biosynthetic approach to the structure of eumelanins—The isolation of oligomers from 5,6-dihydroxy-1-methylindole. Tetrahedron 1986, 42, 2083–2088. [Google Scholar] [CrossRef]
- Panzella, L.; Pezzella, A.; Napolitano, A.; d’Ischia, M. The first 5,6-dihydroxyindole tetramer by oxidation of 5,5′,6,6′-tetrahydroxy-2,4′-biindolyl and an unexpected issue of positional reactivity en route to eumelanin-related polymers. Org. Lett. 2007, 72, 9225–9230. [Google Scholar] [CrossRef] [PubMed]
- Pezzella, A.; Panzella, L.; Natangelo, A.; Arzillo, M.; Napolitano, A.; d’Ischia, M. 5,6-dihydroxyindole tetramers with “anomalous” interunit bonding patterns by oxidative coupling of 5,5′,6,6′-tetrahydroxy-2,7′-biindolyl: Emerging complexities on the way toward an improved model of eumelanin buildup. J. Org. Chem. 2007, 72, 9225–9230. [Google Scholar] [CrossRef] [PubMed]
- Pezzella, A.; Vogna, D.; Prota, G. Atropoisomeric melanin intermediates by oxidation of the melanogenic precursor 5,6-dihydroxyindole-2-carboxylic acid under biomimetic conditions. Tetrahedron 2002, 58, 3681–3687. [Google Scholar] [CrossRef]
- Pezzella, A.; Vogna, D.; Prota, G. Synthesis of optically active tetrameric melanin intermediates by oxidation of the melanogenic precursor 5,6-dihydroxyindole-2-carboxylic acid under biomimetic conditions. Tetrahedron Asymmetry 2003, 14, 1133–1140. [Google Scholar] [CrossRef]
- Kaxiras, E.; Tsolakidis, A.; Zonios, G.; Meng, S. Structural model of eumelanin. Phys. Rev. Lett. 2006, 97, 218102. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Kaxiras, E. Theoretical models of eumelanin protomolecules and their optical properties. Biophys. J. 2008, 94, 2095–2105. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-T.; Ball, V.; de Almeida Gracio, J.J.; Singh, M.K.; Toniazzo, V.R.; Ruch, D.; Buehler, M.J. Self-assembly of tetramers of 5,6-dihydroxyindole explains the primary physical properties of eumelanin: Experiment, simulation, and design. ACS Nano 2013, 7, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- Crescenzi, O.; d’Ischia, M.; Napolitano, A. Kaxiras’s porphyrin: DFT modeling of redox-tuned optical and electronic properties in a theoretically designed catechol-based bioinspired platform. Biomimetics 2017, 2, 21. [Google Scholar] [CrossRef]
- Liebscher, J.; Mrówczyński, R.; Scheidt, H.A.; Filip, C.; Hădade, N.D.; Turcu, R.; Bende, A.; Beck, S. Structure of polydopamine: A never-ending story? Langmuir 2013, 29, 10539–10548. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Yu, X.; Liu, Y.; Shi, Z.; Liu, H.; Nie, Z.; Wu, D.; Jin, Z. Folic acid-polydopamine nanofibers show enhanced ordered-stacking via π-π interactions. Soft Matter 2015, 11, 4621–4629. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Khetan, A.; Wu, W.; Chun, S.-E.; Viswanathan, V.; Whitacre, J.F.; Bettinger, C.J. Evidence of porphyrin-like structures in natural melanin pigments using electrochemical fingerprinting. Adv. Mater. 2016, 28, 3173–3180. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, M.L.; Micillo, R.; Panzella, L.; Crescenzi, O.; Oscurato, S.L.; Maddalena, P.; Napolitano, A.; Ball, V.; d’Ischia, M. The structural basis of polydopamine film formation: Probing 5,6-dihydroxyindole-based eumelanin type units and the porphyrin issue. ACS Appl. Mater. Interfaces 2018, 10, 7670–7680. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.L.; Powell, B.J.; Meredith, P. Chemical and structural disorder in eumelanins: A possible explanation for broadband absorbance. Biophys. J. 2006, 90, 743–752. [Google Scholar] [CrossRef] [PubMed]
- D’ischia, M.; Napolitano, A.; Pezzella, A.; Meredith, P.; Sarna, T. Chemical and structural diversity in eumelanins: Unexplored bio-optoelectronic materials. Angew. Chem. Int. Ed. 2009, 48, 3914–3921. [Google Scholar] [CrossRef] [PubMed]
- Pezzella, A.; Iadonisi, A.; Valerio, S.; Panzella, L.; Napolitano, A.; Adinolfi, M.; d’Ischia, M. Disentangling eumelanin “black chromophore”: Visible absorption changes as signatures of oxidation state- and aggregation-dependent dynamic interactions in a model water-soluble 5,6-dihydroxyindole polymer. J. Am. Chem. Soc. 2009, 131, 15270–15275. [Google Scholar] [CrossRef] [PubMed]
- Ascione, L.; Pezzella, A.; Ambrogi, V.; Carfagna, C.; d’Ischia, M. Intermolecular π-electron perturbations generate extrinsic visible contributions to eumelanin black chromophore in model polymers with interrupted interring conjugation. Photochem. Photobiol. 2013, 89, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Arzillo, M.; Mangiapia, G.; Pezzella, A.; Heenan, R.K.; Radulescu, A.; Paduano, L.; d’Ischia, M. Eumelanin buildup on the nanoscale: Aggregate growth/assembly and visible absorption development in biomimetic 5,6-dihydroxyindole polymerization. Biomacromolecules 2012, 13, 2379–2390. [Google Scholar] [CrossRef] [PubMed]
- Micillo, R.; Panzella, L.; Iacomino, M.; Prampolini, G.; Cacelli, I.; Ferretti, A.; Crescenzi, O.; Koike, K.; Napolitano, A.; d’Ischia, M. Eumelanin broadband absorption develops from aggregation-modulated chromophore interactions under structural and redox control. Sci. Rep. 2017, 7, 41532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pezzella, A.; Panzella, L.; Crescenzi, O.; Napolitano, A.; Navaratnam, S.; Edge, R.; Land, E.J.; Barone, V.; d’Ischia, M. Short-lived quinonoid species from 5,6-dihydroxyindole dimers en route to eumelanin polymers: Integrated chemical, pulse radiolytic, and quantum mechanical investigation. J. Am. Chem. Soc. 2006, 128, 15490–15498. [Google Scholar] [CrossRef] [PubMed]
- Pezzella, A.; Panzella, L.; Crescenzi, O.; Napolitano, A.; Navaratnam, S.; Edge, R.; Land, E.J.; Barone, V.; d’Ischia, M. Lack of visible chromophore development in the pulse radiolysis oxidation of 5,6-dihydroxyindole-2-carboxylic acid oligomers: DFT investigation and implications for eumelanin absorption properties. J. Org. Chem. 2009, 74, 3727–3734. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Liu, X.M.; Dai, X.; Zhou, Q.; Lei, T.C.; Beermann, F.; Wakamatsu, K.; Xu, S.Z. Regulation of DHICA-mediated antioxidation by dopachrome tautomerase: Implication for skin photoprotection against UVA radiation. Free Radic. Biol. Med. 2010, 48, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Gentile, G.; D’Errico, G.; Della Vecchia, N.F.; Errico, M.E.; Napolitano, A.; Carfagna, C.; d’Ischia, M. Atypical structural and π-electron features in the melanin polymer from the major human melanogen underpin superior free radical scavenger properties. Angew. Chem. Int. Ed. 2013, 52, 12684–12687. [Google Scholar] [CrossRef] [PubMed]
- D’Ischia, M.; Crescenzi, O.; Pezzella, A.; Arzillo, M.; Panzella, L.; Napolitano, A.; Barone, V. Structural effects on the electronic absorption of 5,6-dihydroxyindole oligomers: The potential of an integrated experimental and DFT approach to model eumelanin optical properties. Photochem. Photobiol. 2008, 84, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Nagase, S.; Tsuchiya, M.; Matsui, T.; Shibuichi, S.; Tsujimura, H.; Masukawa, Y.; Satoh, N.; Itou, T.; Koike, K.; Tsujii, K. Characterization of curved hair of Japanese women with reference to internal structures and amino acid composition. J. Cosmet. Sci. 2008, 59, 317–332. [Google Scholar] [PubMed]
- Bryson, W.G.; Harland, D.P.; Caldwell, J.P.; Vernon, J.A.; Walls, R.J.; Woods, J.L.; Nagase, S.; Itou, T.; Koike, K. Cortical cell types and intermediate filament arrangements correlate with fiber curvature in Japanese human hair. J. Struct. Biol. 2008, 166, 46–58. [Google Scholar] [CrossRef] [PubMed]
- De Gàlvez, M.V.; Aguilera, J.; Bernabó, J.L.; Sánchez-Roldán, C.; Herrera-Ceballos, E. Human hair as a natural sun protection agent: A quantitative study. Photochem. Photobiol. 2015, 91, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Dario, M.F.; Baby, A.R.; Velasco, M.V. Effects of solar radiation on hair and photoprotection. J. Photochem. Photobiol. B 2015, 153, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Miyamura, Y.; Wolber, R.; Smuda, C.; Reinhold, W.; Liu, H.; Kolbe, L.; Hearing, V.J. Regulation of human skin pigmentation in situ by repetitive UV exposure: Molecular characterization of responses to UVA and/or UVB. J. Investig. Dermatol. 2010, 130, 1685–1696. [Google Scholar] [CrossRef] [PubMed]
- Wolber, R.; Schlenz, K.; Wakamatsu, K.; Smuda, C.; Nakanishi, Y.; Hearing, V.J.; Ito, S. Pigmentation effects of solar-simulated radiation as compared with UVA and UVB radiation. Pigment Cell Melanoma Res. 2008, 21, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Miyamura, Y.; Coelho, S.G.; Wolber, R.; Miller, S.A.; Wakamatsu, K.; Zmudzka, B.Z.; Ito, S.; Smuda, C.; Passeron, T.; et al. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment Cell Res. 2007, 20, 2–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Routaboul, C.; Denis, A.; Vinche, A. Immediate pigment darkening: Description, kinetic and biological function. Eur. J. Dermatol. 1999, 9, 95–99. [Google Scholar] [PubMed]
- Maeda, K.; Hatao, M. Involvement of photooxidation of melanogenic precursors in prolonged pigmentation induced by ultraviolet A. J. Investig. Dermatol. 2004, 122, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Young, A.R. Acute effects of UVR on human eyes and skin. Prog. Biophys. Mol. Biol. 2006, 92, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, T.; Yamaguchi, Y.; Batzer, J.; Coelho, S.G.; Zmudzka, B.Z.; Miller, S.A.; Wolber, R.; Beer, J.Z.; Hearing, V.J. Mechanisms of skin tanning in different racial/ethnic groups in response to ultraviolet radiation. J. Investig. Dermatol. 2005, 124, 1326–1332. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, E.K.; Granter, S.R.; Fisher, D.E. Mechanisms of hair graying: Incomplete melanocyte stem cell maintenance in the niche. Science 2005, 5710, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Bolduc, C.; Shapiro, J. Hair care products: Waving, straightening, conditioning, and coloring. Clin. Dermatol. 2001, 19, 431–436. [Google Scholar] [CrossRef]
- De Groot, A.C. Side-effects of henna and semi-permanent ‘black henna’ tattoos: A full review. Contact Dermat. 2013, 69, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.K. Hair cosmetics. Dermatol. Clin. 1991, 9, 19–27. [Google Scholar] [PubMed]
- Pang, S.; Fiume, M.Z. Final report on the safety assessment of ammonium, potassium, and sodium persulfate. Int. J. Toxicol. 2001, 20, 7–21. [Google Scholar] [PubMed]
- Hamann, D.; Yazar, K.; Hamann, C.R.; Thyssen, J.P.; Liden, C. p-Phenylenediamine and other allergens in hair dye products in the United States: A consumer exposure study. Contact Dermat. 2014, 70, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Mensing, T.; Marek, W.; Raulf-Heimsoth, M.; Baur, X. Acute exposure to hair bleach causes airway hyperresponsiveness in a rabbit model. Eur. Respir. J. 1998, 12, 1371–1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, M.S.; Lee, C.M.; Jeong, W.J.; Kim, S.J.; Lee, K.Y. Significant damage of the skin and hair following hair bleaching. J. Dermatol. 2010, 37, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.C.; Sawlani, K.K.; Singh, K. Paraphenylenediamine poisoning. Niger. J. Clin. Pract. 2013, 16, 258–259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kim, C.; Zheng, T. Hair dye use and risk of human cancer. Front. Biosci. Elite Ed. 2012, E4, 516–528. [Google Scholar] [CrossRef]
- Tanamachi, H.; Tokunaga, S.; Tanji, N.; Oguri, M.; Inoue, S.J. 18-MEA and hair appearance. J. Cosmet. Sci. 2010, 61, 147–160. [Google Scholar] [PubMed]
- Lee, Y.; Kim, Y.-D.; Hyun, H.-J.; Pi, L.; Jin, X.; Lee, W.-S. Hair shaft damage from heat and drying time of hair dryer. Ann. Dermatol. 2011, 23, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Im, K.M.; Kim, T.-W.; Jeon, J.-R. Metal-chelation-assisted deposition of polydopamine on human hair: A ready-to-use eumelanin-based hair dyeing methodology. ACS Biomater. Sci. Eng. 2017, 3, 628–636. [Google Scholar] [CrossRef]
- Obata, H.; Ishida, H.; Hata, Y.; Kawato, A.; Abe, Y.; Akao, T.; Akita, O.; Ichishima, E. Cloning of a novel tyrosinase-encoding gene (melB) from Aspergillus oryzae and its overexpression in solid-state culture (Rice Koji). J. Biosci. Bioeng. 2004, 97, 400–405. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yamanaka, H.; Hata, Y.; Ebato, A.; Koike, K. Development of the novel hair coloring system using enzymatically prepared melanin precursors by Aspergillus oryzae tyrosinase. Seibutsu-Kogaku Kaishi 2012, 90, 115–121. [Google Scholar]
- Battie, C.; Jitsukawa, S.; Bernerd, F.; Del Bino, S.; Marionnet, C.; Verschoore, M. New insights in photoaging, UVA induced damage and skin types. Exp. Dermatol. 2014, 23, 7–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szewczyk, G.; Zadlo, A.; Sarna, M.; Ito, S.; Wakamatsu, K.; Sarna, T. Aerobic photoreactivity of synthetic eumelanins and pheomelanins: Generation of singlet oxygen and superoxide anion. Pigment Cell Melanoma Res. 2016, 29, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Micillo, R.; Iacomino, M.; Perfetti, M.; Panzella, L.; Koike, K.; D’Errico, G.; d’Ischia, M.; Napolitano, A. Unexpected impact of esterification on the antioxidant activity and (photo)stability of a eumelanin from 5,6-dihydroxyindole-2-carboxylic acid. Pigment Cell Melanoma Res. 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panzella, L.; Ebato, A.; Napolitano, A.; Koike, K. The Late Stages of Melanogenesis: Exploring the Chemical Facets and the Application Opportunities. Int. J. Mol. Sci. 2018, 19, 1753. https://doi.org/10.3390/ijms19061753
Panzella L, Ebato A, Napolitano A, Koike K. The Late Stages of Melanogenesis: Exploring the Chemical Facets and the Application Opportunities. International Journal of Molecular Sciences. 2018; 19(6):1753. https://doi.org/10.3390/ijms19061753
Chicago/Turabian StylePanzella, Lucia, Atsuko Ebato, Alessandra Napolitano, and Kenzo Koike. 2018. "The Late Stages of Melanogenesis: Exploring the Chemical Facets and the Application Opportunities" International Journal of Molecular Sciences 19, no. 6: 1753. https://doi.org/10.3390/ijms19061753