Antibacterial Free Fatty Acids and Monoglycerides: Biological Activities, Experimental Testing, and Therapeutic Applications
Abstract
:1. Introduction
2. Antimicrobial Lipids
2.1. Classifications
2.2. Spectrum of Antibacterial Activity
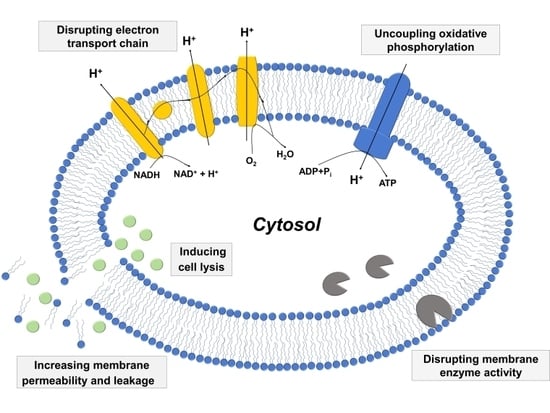
2.3. Mechanisms of Antibacterial Activity
2.3.1. Increased Membrane Permeability and Cell Lysis
2.3.2. Disrupting Electron Transport Chain and Uncoupling Oxidative Phosphorylation
2.3.3. Inhibiting Activity of Bacterial Enzymes
3. Experimental Approaches to Characterize Antimicrobial Lipids
3.1. Anti-Infective Evaluation of Bacterial Specimens
3.1.1. Growth Inhibition Assays
3.1.2. Infectivity Assays
3.1.3. Electron Microscopy
3.2. Biophysical Approaches with Model Membrane Platforms
3.2.1. Solution-Phase Liposomes
3.2.2. Giant Unilamellar Vesicle
3.2.3. Supported Lipid Bilayers
4. Examples of Therapeutic Applications
4.1. Systemic Treatment of Stomach Infection
4.2. Topical Treatment of Skin Infection
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Antonietti, M.; Förster, S. Vesicles and liposomes: A self-assembly principle beyond lipids. Adv. Mater. 2003, 15, 1323–1333. [Google Scholar] [CrossRef]
- Ninham, B.W.; Larsson, K.; Nostro, P.L. Two sides of the coin. Part 2. Colloid and surface science meets real biointerfaces. Colloids Surf. B Biointerfaces 2017, 159, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Mukai, M.; Regen, S.L. Lipid raft formation driven by push and pull forces. Bull. Chem. Soc. Japan 2017, 90, 1083–1087. [Google Scholar] [CrossRef]
- Ramanathan, M.; Shrestha, L.K.; Mori, T.; Ji, Q.; Hill, J.P.; Ariga, K. Amphiphile nanoarchitectonics: From basic physical chemistry to advanced applications. Phys. Chem. Chem. Phys. 2013, 15, 10580–10611. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, M.; Yoshimoto, K.; Sisido, M.; Ariga, K. Chemistry can make strict and fuzzy controls for bio-systems: DNA nanoarchitectonics and cell-macromolecular nanoarchitectonics. Bull. Chem. Soc. Japan 2017, 90, 967–1004. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.; Wertheim, H.F.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H. Antibiotic resistance—The need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef]
- Aminov, R.I. A brief history of the antibiotic era: Lessons learned and challenges for the future. Front. Microbiol. 2010, 1, 134. [Google Scholar] [CrossRef] [PubMed]
- Alanis, A.J. Resistance to antibiotics: Are we in the post-antibiotic era? Arch. Med. Res. 2005, 36, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Thormar, H. Lipids and Essential Oils as Antimicrobial Agents; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Chen, Y.E.; Tsao, H. The skin microbiome: Current perspectives and future challenges. J. Am. Acad. Dermatol. 2013, 69, 143.e3–155.e3. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Gallo, R.L. Antimicrobial peptides: Old molecules with new ideas. J. Investig. Dermatol. 2012, 132, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabara, J.J.; Swieczkowski, D.M.; Conley, A.J.; Truant, J.P. Fatty acids and derivatives as antimicrobial agents. Antimicrob. Agents Chemother. 1972, 2, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Kabara, J.; Vrable, R.; Jie, M.L.K. Antimicrobial lipids: Natural and synthetic fatty acids and monoglycerides. Lipids 1977, 12, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Kabara, J.J. Antimicrobial agents derived from fatty acids. J. Ame. Oil Chem. Soc. 1984, 61, 397–403. [Google Scholar] [CrossRef]
- Kabara, J.J. Structure-function relationships of surfactants as antimicrobial agents. J. Soc. Cosmet. Chem. 1978, 29, 733–741. [Google Scholar]
- Kabara, J.J. GRAS antimicrobial agents for cosmetic products. J. Soc. Cosmet. Chem. 1980, 31, 1–10. [Google Scholar]
- Kitahara, T.; Koyama, N.; Matsuda, J.; Aoyama, Y.; Hirakata, Y.; Kamihira, S.; Kohno, S.; Nakashima, M.; Sasaki, H. Antimicrobial activity of saturated fatty acids and fatty amines against methicillin-resistant Staphylococcus aureus. Biol. Pharm. Bull. 2004, 27, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Kao, M.C.; Fang, J.-Y.; Zouboulis, C.C.; Zhang, L.; Gallo, R.L.; Huang, C.-M. Antimicrobial property of lauric acid against Propionibacterium acnes: Its therapeutic potential for inflammatory acne vulgaris. J. Investig. Dermatol. 2009, 129, 2480–2488. [Google Scholar] [CrossRef] [PubMed]
- Schlievert, P.M.; Peterson, M.L. Glycerol monolaurate antibacterial activity in broth and biofilm cultures. PLoS ONE 2012, 7, e40350. [Google Scholar] [CrossRef] [PubMed]
- Skřivanová, E.; Molatová, Z.; Marounek, M. Effects of caprylic acid and triacylglycerols of both caprylic and capric acid in rabbits experimentally infected with enteropathogenic Escherichia coli O103. Vet. Microbiol. 2008, 126, 372–376. [Google Scholar] [CrossRef] [PubMed]
- P Desbois, A. Potential applications of antimicrobial fatty acids in medicine, agriculture and other industries. Recent Patents Anti-Infect. Drug Discov. 2012, 7, 111–122. [Google Scholar] [CrossRef]
- Maag, H. Fatty acid derivatives: Important surfactants for household, cosmetic and industrial purposes. J. Am. Oil Chem. Soc. 1984, 61, 259–267. [Google Scholar] [CrossRef]
- Heerklotz, H. Interactions of surfactants with lipid membranes. Quart. Rev. Biophys. 2008, 41, 205–264. [Google Scholar] [CrossRef] [PubMed]
- Speert, D.P.; Wannamaker, L.W.; Gray, E.D.; Clawson, C.C. Bactericidal effect of oleic acid on group A streptococci: Mechanism of action. Infect. Immun. 1979, 26, 1202–1210. [Google Scholar] [PubMed]
- Knapp, H.R.; Melly, M.A. Bactericidal effects of polyunsaturated fatty acids. J. Infect. Dis. 1986, 154, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-L.; Johnson, E.A. Inhibition of Listeria monocytogenes by fatty acids and monoglycerides. Appl. Environ. Microbiol. 1992, 58, 624–629. [Google Scholar] [PubMed]
- Bergsson, G.; Arnfinnsson, J.; Karlsson, S.M.; Steingrímsson, Ó.; Thormar, H. In vitro inactivation of Chlamydia trachomatis by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 1998, 42, 2290–2294. [Google Scholar] [PubMed]
- Bergsson, G.; Arnfinnsson, J.; SteingrÍmsson, Ó.; Thormar, H. Killing of Gram-positive cocci by fatty acids and monoglycerides. APMIS 2001, 109, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Skřivanová, E.; Marounek, M.; Dlouha, G.; Kaňka, J. Susceptibility of Clostridium perfringens to C2–C18 fatty acids. Lett. Appl. Microbiol. 2005, 41, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Bajpai, V.; Kim, H.; Kang, S. Antibacterial activity of eicosapentaenoic acid (EPA) against foodborne and food spoilage microorganisms. LWT-Food Sci. Technol. 2007, 40, 1515–1519. [Google Scholar] [CrossRef]
- Lonchin, S.; Luisi, P.L.; Walde, P.; Robinson, B.H. A matrix effect in mixed phospholipid/fatty acid vesicle formation. J. Phys. Chem. B 1999, 103, 10910–10916. [Google Scholar] [CrossRef]
- Berclaz, N.; Müller, M.; Walde, P.; Luisi, P.L. Growth and transformation of vesicles studied by ferritin labeling and cryotransmission electron microscopy. J. Phys. Chem. B 2001, 105, 1056–1064. [Google Scholar] [CrossRef]
- Berclaz, N.; Blöchliger, E.; Müller, M.; Luisi, P.L. Matrix effect of vesicle formation as investigated by cryotransmission electron microscopy. J. Phys. Chem. B 2001, 105, 1065–1071. [Google Scholar] [CrossRef]
- Rasi, S.; Mavelli, F.; Luisi, P.L. Cooperative micelle binding and matrix effect in oleate vesicle formation. J. Phys. Chem. B 2003, 107, 14068–14076. [Google Scholar] [CrossRef]
- Chungcharoenwattana, S.; Ueno, M. Size control of mixed egg yolk phosphatidylcholine (EggPC)/oleate vesicles. Chem. Pharm. Bull. 2004, 52, 1058–1062. [Google Scholar] [CrossRef] [PubMed]
- Chungcharoenwattana, S.; Kashiwagi, H.; Ueno, M. Effect of preformed egg phosphatidylcholine vesicles on spontaneous vesiculation of oleate micelles. Colloid Polym. Sci. 2005, 283, 1180–1189. [Google Scholar] [CrossRef]
- Chungcharoenwattana, S.; Ueno, M. New vesicle formation upon oleate addition to preformed vesicles. Chem. Pharm. Bull. 2005, 53, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Rogerson, M.L.; Robinson, B.H.; Bucak, S.; Walde, P. Kinetic studies of the interaction of fatty acids with phosphatidylcholine vesicles (liposomes). Colloids Surf. B Biointerfaces 2006, 48, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Peterlin, P.; Arrigler, V.; Kogej, K.; Svetina, S.; Walde, P. Growth and shape transformations of giant phospholipid vesicles upon interaction with an aqueous oleic acid suspension. Chem. Phys. Lipids 2009, 159, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Mally, M.; Peterlin, P.; Svetina, S. Partitioning of oleic acid into phosphatidylcholine membranes is amplified by strain. J. Phys. Chem. B 2013, 117, 12086–12094. [Google Scholar] [CrossRef] [PubMed]
- Thid, D.; Benkoski, J.J.; Svedhem, S.; Kasemo, B.; Gold, J. DHA-induced changes of supported lipid membrane morphology. Langmuir 2007, 23, 5878–5881. [Google Scholar] [CrossRef] [PubMed]
- Flynn, K.R.; Martin, L.L.; Ackland, M.L.; Torriero, A.A. Real-time quartz crystal microbalance monitoring of free docosahexaenoic acid interactions with supported lipid bilayers. Langmuir 2016, 32, 11717–11727. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, M.; Sutherland, D.S.; Sundh, M.; Mygind, T.; Meyer, R.L. Antimicrobial mechanism of monocaprylate. Appl. Environ. Microbiol. 2012, 78, 2957–2965. [Google Scholar] [CrossRef] [PubMed]
- Barratt, M. Quantitative structure-activity relationships (QSARs) for skin corrosivity of organic acids, bases and phenols: Principal components and neural network analysis of extended datasets. Toxicol. In Vitro 1996, 10, 85–94. [Google Scholar] [CrossRef]
- Osborn, H.; Akoh, C. Structured lipids-novel fats with medical, nutraceutical, and food applications. Compr. Rev. Food Sci. Food Saf. 2002, 1, 110–120. [Google Scholar] [CrossRef]
- Bazinet, R.P.; Layé, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014, 15, 771. [Google Scholar] [CrossRef] [PubMed]
- Kodicek, E.; Worden, A. The effect of unsaturated fatty acids on Lactobacillus helveticus and other Gram-positive micro-organisms. Biochem. J. 1945, 39, 78. [Google Scholar] [CrossRef] [PubMed]
- Galbraith, H.; Miller, T.; Paton, A.; Thompson, J. Antibacterial activity of long chain fatty acids and the reversal with calcium, magnesium, ergocalciferol and cholesterol. J. Appl. Microbiol. 1971, 34, 803–813. [Google Scholar] [CrossRef]
- Saito, H.; Tomioka, H.; Yoneyama, T. Growth of group IV mycobacteria on medium containing various saturated and unsaturated fatty acids. Antimicrob. Agents Chemother. 1984, 26, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Feldlaufer, M.; Knox, D.; Lusby, W.; Shimanuki, H. Antimicrobial activity of fatty acids against Bacillus larvae, the causative agent of American foulbrood disease. Apidologie 1993, 24, 95–99. [Google Scholar] [CrossRef]
- Wille, J.; Kydonieus, A. Palmitoleic acid isomer (C16: 1Δ6) in human skin sebum is effective against gram-positive bacteria. Skin Pharmacol. Physiol. 2003, 16, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Q.; O’Connor, C.J.; Roberton, A.M. Antibacterial actions of fatty acids and monoglycerides against Helicobacter pylori. FEMS Immunol. Med. Microbiol. 2003, 36, 9–17. [Google Scholar] [CrossRef]
- Heczko, P.; Lütticken, R.; Hryniewicz, W.; Neugebauer, M.; Pulverer, G. Susceptibility of Staphylococcus aureus and group A, B, C, and G streptococci to free fatty acids. J. Clin. Microbiol. 1979, 9, 333–335. [Google Scholar] [PubMed]
- Thormar, H.; Hilmarsson, H.; Bergsson, G. Stable concentrated emulsions of the 1-monoglyceride of capric acid (monocaprin) with microbicidal activities against the food-borne bacteria Campylobacter jejuni, Salmonella spp., and Escherichia coli. Appl. Environ. Microbiol. 2006, 72, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Rohrer, L.; Winterhalter, K.; Eckert, J.; Köhler, P. Killing of Giardia lamblia by human milk is mediated by unsaturated fatty acids. Antimicrob. Agents Chemother. 1986, 30, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Thormar, H.; Isaacs, C.E.; Brown, H.R.; Barshatzky, M.R.; Pessolano, T. Inactivation of enveloped viruses and killing of cells by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 1987, 31, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Hilmarsson, H.; Larusson, L.; Thormar, H. Virucidal effect of lipids on visna virus, a lentivirus related to HIV. Arch. Virol. 2006, 151, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Kanetsuna, F. Bactericidal effect of fatty acids on mycobacteria, with particular reference to the suggested mechanism of intracellular killing. Microbiol. Immunol. 1985, 29, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Greenway, D.; Dyke, K. Mechanism of the inhibitory action of linoleic acid on the growth of Staphylococcus aureus. Microbiology 1979, 115, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Petschow, B.W.; Batema, R.P.; Ford, L.L. Susceptibility of Helicobacter pylori to bactericidal properties of medium-chain monoglycerides and free fatty acids. Antimicrob. Agents Chemother. 1996, 40, 302–306. [Google Scholar] [PubMed]
- Bergsson, G.; Steingrímsson, Ó.; Thormar, H. In vitro susceptibilities of Neisseria gonorrhoeae to fatty acids and monoglycerides. Antimicrob. Agents Chemother. 1999, 43, 2790–2792. [Google Scholar] [PubMed]
- Bergsson, G.; Steingrímsson, Ó.; Thormar, H. Bactericidal effects of fatty acids and monoglycerides on Helicobacter pylori. Int. J. Antimicrob. Agents 2002, 20, 258–262. [Google Scholar] [CrossRef]
- Marounek, M.; Skřivanová, E.; Rada, V. Susceptibility of Escherichia coli to C2–C18 fatty acids. Folia Microbiologica 2003, 48, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Skřivanová, E.; Savka, O.; Marounek, M. In vitro effect of C2–C18 fatty acids on salmonellas. Folia microbiologica 2004, 49, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Benkendorff, K.; Davis, A.R.; Rogers, C.N.; Bremner, J.B. Free fatty acids and sterols in the benthic spawn of aquatic molluscs, and their associated antimicrobial properties. J. Exp. Mar. Biol. Ecol. 2005, 316, 29–44. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Kim, Y.-S.; Shin, D.-H. Antimicrobial synergistic effect of linolenic acid and monoglyceride against Bacillus cereus and Staphylococcus aureus. J. Agric. Food Chem. 2002, 50, 2193–2199. [Google Scholar] [CrossRef] [PubMed]
- Kollanoor, A.; Vasudevan, P.; Nair, M.K.M.; Hoagland, T.; Venkitanarayanan, K. Inactivation of bacterial fish pathogens by medium-chain lipid molecules (caprylic acid, monocaprylin and sodium caprylate). Aquac. Res. 2007, 38, 1293–1300. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, H.; Cui, Y.; Zhao, G.; Feng, F. Antibacterial interactions of monolaurin with commonly used antimicrobials and food components. J. Food Sci. 2009, 74. [Google Scholar] [CrossRef] [PubMed]
- Doležalová, M.; Janiš, R.; Svobodová, H.; Kašpárková, V.; Humpolíček, P.; Krejčí, J. Antimicrobial properties of 1-monoacylglycerols prepared from undecanoic (C11:0) and undecenoic (C11:1) acid. Eur. J. Lipid Sci. Technol. 2010, 112, 1106–1114. [Google Scholar] [CrossRef]
- Marounek, M.; Putthana, V.; Benada, O.; Lukešová, D. Antimicrobial activities of medium-chain fatty acids and monoacylglycerols on Cronobacter sakazakii DBM 3157T and Cronobacter malonaticus DBM 3148. Czech J. Food Sci. 2012, 30, 573–580. [Google Scholar] [CrossRef]
- Annamalai, T.; Nair, M.K.M.; Marek, P.; Vasudevan, P.; Schreiber, D.; Knight, R.; Hoagland, T.; Venkitanarayanan, K. In vitro inactivation of Escherichia coli O157: H7 in bovine rumen fluid by caprylic acid. J. Food Prot. 2004, 67, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.K.M.; Abouelezz, H.; Hoagland, T.; Venkitanarayanan, K. Antibacterial effect of monocaprylin on Escherichia coli O157: H7 in apple juice. J. Food Prot. 2005, 68, 1895–1899. [Google Scholar] [CrossRef] [PubMed]
- Amalaradjou, M.A.R.; Annamalai, T.; Marek, P.; Rezamand, P.; Schreiber, D.; Hoagland, T.; Venkitanarayanan, K. Inactivation of Escherichia coli O157: H7 in cattle drinking water by sodium caprylate. J. Food Prot. 2006, 69, 2248–2252. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Rhee, M. Marked synergistic bactericidal effects and mode of action of medium-chain fatty acids in combination with organic acids against Escherichia coli O157: H7. Appl. Environ. Microbiol. 2013, 79, 6552–6560. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Rhee, M. Synergistic antimicrobial activity of caprylic acid in combination with citric acid against both Escherichia coli O157: H7 and indigenous microflora in carrot juice. Food Microbiol. 2015, 49, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, P.; Marek, P.; Nair, M.K.M.; Annamalai, T.; Darre, M.; Khan, M.; Venkitanarayanan, K. In vitro inactivation of Salmonella enteritidis in autoclaved chicken cecal contents by caprylic acid. J. Appl. Poult. Res. 2005, 14, 122–125. [Google Scholar] [CrossRef]
- Chang, S.-s.; Redondo-Solano, M.; Thippareddi, H. Inactivation of Escherichia coli O157: H7 and Salmonella spp. on alfalfa seeds by caprylic acid and monocaprylin. Int. J. Food Microbiol. 2010, 144, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.K.M.; Vasudevan, P.; Hoagland, T.; Venkitanarayanan, K. Inactivation of Escherichia coli O157: H7 and Listeria monocytogenes in milk by caprylic acid and monocaprylin. Food Microbiol. 2004, 21, 611–616. [Google Scholar] [CrossRef]
- Garcia, M.; Amalaradjou, M.A.R.; Nair, M.K.M.; Annamalai, T.; Surendranath, S.; Lee, S.; Hoagland, T.; Dzurec, D.; Faustman, C.; Venkitanarayanan, K. Inactivation of Listeria monocytogenes on frankfurters by monocaprylin alone or in combination with acetic acid. J. Food Prot. 2007, 70, 1594–1599. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.; Joy, J.; Vasudevan, P.; Hinckley, L.; Hoagland, T.; Venkitanarayanan, K. Antibacterial effect of caprylic acid and monocaprylin on major bacterial mastitis pathogens. J. Dairy Sci. 2005, 88, 3488–3495. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Schlievert, P.M.; Anderson, M.J.; Fair, C.L.; Schaefers, M.M.; Muthyala, R.; Peterson, M.L. Glycerol monolaurate and dodecylglycerol effects on Staphylococcus aureus and toxic shock syndrome toxin-1 in vitro and in vivo. PLoS ONE 2009, 4, e7499. [Google Scholar] [CrossRef] [PubMed]
- Preuss, H.G.; Echard, B.; Dadgar, A.; Talpur, N.; Manohar, V.; Enig, M.; Bagchi, D.; Ingram, C. Effects of essential oils and monolaurin on Staphylococcus aureus: In vitro and in vivo studies. Toxicol. Mech. Methods 2005, 15, 279–285. [Google Scholar] [CrossRef] [PubMed]
- McLay, J.; Kennedy, M.; O’Rourke, A.-L.; Elliot, R.; Simmonds, R. Inhibition of bacterial foodborne pathogens by the lactoperoxidase system in combination with monolaurin. Int. J. Food Microbiol. 2002, 73, 1–9. [Google Scholar] [CrossRef]
- Choi, M.; Kim, S.; Lee, N.; Rhee, M. New decontamination method based on caprylic acid in combination with citric acid or vanillin for eliminating Cronobacter sakazakii and Salmonella enterica serovar Typhimurium in reconstituted infant formula. Int. J. Food Microbiol. 2013, 166, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Rhee, M. Highly enhanced bactericidal effects of medium chain fatty acids (caprylic, capric, and lauric acid) combined with edible plant essential oils (carvacrol, eugenol, β-resorcylic acid, trans-cinnamaldehyde, thymol, and vanillin) against Escherichia coli O157: H7. Food Control 2016, 60, 447–454. [Google Scholar]
- Hovorková, P.; Laloučková, K.; Skřivanová, E. Determination of in vitro antibacterial activity of plant oils containing medium-chain fatty acids against gram-positive pathogenic and gut commensal bacteria. Czech J. Anim. Sci. 2018, 63, 119–125. [Google Scholar] [CrossRef]
- Anacarso, I.; Quartieri, A.; De Leo, R.; Pulvirenti, A. Evaluation of the antimicrobial activity of a blend of monoglycerides against Escherichia coli and Enterococci with multiple drug resistance. Arch. Microbiol. 2018, 200, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, H.; Goto, Y.; Aida, M.; Hosokawa, M.; Takahashi, K. Antibacterial activity against cariogenic bacteria and inhibition of insoluble glucan production by free fatty acids obtained from dried Gloiopeltis furcata. Fish. Sci. 1999, 65, 129–132. [Google Scholar] [CrossRef]
- Won, S.-R.; Hong, M.-J.; Kim, Y.-M.; Li, C.Y.; Kim, J.-W.; Rhee, H.-I. Oleic acid: An efficient inhibitor of glucosyltransferase. FEBS Lett. 2007, 581, 4999–5002. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Stevens, M.J.; Neuenschwander, S.; Schwarm, A.; Kreuzer, M.; Bratus-Neuenschwander, A.; Zeitz, J.O. The transcriptome response of the ruminal methanogen Methanobrevibacter ruminantium strain M1 to the inhibitor lauric acid. BMC Res. Notes 2018, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, N.R.; Mehrtens, B.; Xiong, Z.; Kapral, F.; Boardman, J.; Rearick, J. Correlation of carotenoid production, decreased membrane fluidity, and resistance to oleic acid killing in Staphylococcus aureus 18Z. Infect. Immun. 1991, 59, 4332–4337. [Google Scholar] [PubMed]
- Salton, M. The adsorption of cetyltrimethylammonium bromide by bacteria, its action in releasing cellular constituents and its bactericidal effects. Microbiology 1951, 5, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Boyaval, P.; Corre, C.; Dupuis, C.; Roussel, E. Effects of free fatty acids on propionic acid bacteria. Le Lait 1995, 75, 17–29. [Google Scholar] [CrossRef]
- Carson, D.D.; Daneo-Moore, L. Effects of fatty acids on lysis of Streptococcus faecalis. J. Bacteriol. 1980, 141, 1122–1126. [Google Scholar] [PubMed]
- Thompson, L.; Cockayne, A.; Spiller, R. Inhibitory effect of polyunsaturated fatty acids on the growth of Helicobacter pylori: A possible explanation of the effect of diet on peptic ulceration. Gut 1994, 35, 1557–1561. [Google Scholar] [CrossRef] [PubMed]
- Boyer, P.D. The ATP synthase—A splendid molecular machine. Annu. Rev. Biochem. 1997, 66, 717–749. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 1961, 191, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Galbraith, H.; Miller, T. Effect of long chain fatty acids on bacterial respiration and amino acid uptake. J. Appl. Microbiol. 1973, 36, 659–675. [Google Scholar] [CrossRef]
- Sheu, C.W.; Freese, E. Effects of fatty acids on growth and envelope proteins of Bacillus subtilis. J. Bacteriol. 1972, 111, 516–524. [Google Scholar] [PubMed]
- Peters, J.S.; Chin, C.-K. Inhibition of photosynthetic electron transport by palmitoleic acid is partially correlated to loss of thylakoid membrane proteins. Plant Physiol. Biochem. 2003, 41, 117–124. [Google Scholar] [CrossRef]
- Zheng, C.J.; Yoo, J.-S.; Lee, T.-G.; Cho, H.-Y.; Kim, Y.-H.; Kim, W.-G. Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett. 2005, 579, 5157–5162. [Google Scholar] [CrossRef] [PubMed]
- Sado-Kamdem, S.L.; Vannini, L.; Guerzoni, M.E. Effect of α-linolenic, capric and lauric acid on the fatty acid biosynthesis in Staphylococcus aureus. Int. J. Food Microbiol. 2009, 129, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48 (Suppl. 1), S5–S16. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163. [Google Scholar] [CrossRef] [PubMed]
- Ericsson, H.M.; Sherris, J.C. Antibiotic sensitivity testing. Report of an international collaborative study. Acta Pathologica et Microbiologica Scandinavica 1971, 217 (Suppl. 217), 1+. [Google Scholar]
- Arias, C.A.; Murray, B.E. Antibiotic-resistant bugs in the 21st century—A clinical super-challenge. N. Engl. J. Med. 2009, 360, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.K.; Jackman, J.A.; Kim, M.C.; Cho, N.-J. Spectrum of membrane morphological responses to antibacterial fatty acids and related surfactants. Langmuir 2015, 31, 10223–10232. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, I.; Kato, N. Combined effects on antibacterial activity of fatty acids and their esters against gram-negative bacteria. Pharmacol. Effect Lipids 1978, 1978, 15–24. [Google Scholar]
- Dervichian, D. The surface properties of fatty acids and allied substances. Prog. Chem. Fats Other Lipids 1954, 2, 193–242. [Google Scholar] [CrossRef]
- Galbraith, H.; Miller, T. Physicochemical effects of long chain fatty acids on bacterial cells and their protoplasts. J. Appl. Microbiol. 1973, 36, 647–658. [Google Scholar] [CrossRef]
- Miller, R.D.; Brown, K.E.; Morse, S.A. Inhibitory action of fatty acids on the growth of Neisseria gonorrhoeae. Infect. Immun. 1977, 17, 303–312. [Google Scholar] [PubMed]
- French, G. Bactericidal agents in the treatment of MRSA infections—The potential role of daptomycin. J. Antimicrob. Chemother. 2006, 58, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Freundlich, M.M. Origin of the electron microscope. Science 1963, 142, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Ruska, E. The development of the electron microscope and of electron microscopy. Rev. Mod. Phys. 1987, 59, 627. [Google Scholar] [CrossRef]
- Williams, D.B.; Carter, C.B. The transmission electron microscope. In Transmission Electron Microscopy; Springer: Berlin, Germany, 1996; pp. 3–17. [Google Scholar]
- Gabriel, N.E.; Roberts, M.F. Spontaneous formation of stable unilamellar vesicles. Biochemistry 1984, 23, 4011–4015. [Google Scholar] [CrossRef] [PubMed]
- Angelova, M.; Soléau, S.; Méléard, P.; Faucon, F.; Bothorel, P. Preparation of giant vesicles by external AC electric fields. Kinetics and applications. In Trends in Colloid and Interface Science VI; Springer: Berlin, Germany, 1992; pp. 127–131. [Google Scholar]
- Blöchliger, E.; Blocher, M.; Walde, P.; Luisi, P.L. Matrix effect in the size distribution of fatty acid vesicles. J. Phys. Chem. B 1998, 102, 10383–10390. [Google Scholar] [CrossRef]
- Tamba, Y.; Yamazaki, M. Single giant unilamellar vesicle method reveals effect of antimicrobial peptide magainin 2 on membrane permeability. Biochemistry 2005, 44, 15823–15833. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Masum, S.M.; Tanaka, T.; Yamazaki, M. Shape changes of giant unilamellar vesicles of phosphatidylcholine induced by a de novo designed peptide interacting with their membrane interface. Langmuir 2002, 18, 9638–9641. [Google Scholar] [CrossRef]
- Tanaka, T.; Tamba, Y.; Masum, S.M.; Yamashita, Y.; Yamazaki, M. La3+ and Gd3+ induce shape change of giant unilamellar vesicles of phosphatidylcholine. Biochimica et Biophysica Acta (BBA)-Biomembr. 2002, 1564, 173–182. [Google Scholar] [CrossRef]
- Tamba, Y.; Ohba, S.; Kubota, M.; Yoshioka, H.; Yoshioka, H.; Yamazaki, M. Single GUV method reveals interaction of tea catechin (−)-epigallocatechin gallate with lipid membranes. Biophys. J. 2007, 92, 3178–3194. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Sano, R.; Yamashita, Y.; Yamazaki, M. Shape changes and vesicle fission of giant unilamellar vesicles of liquid-ordered phase membrane induced by lysophosphatidylcholine. Langmuir 2004, 20, 9526–9534. [Google Scholar] [CrossRef] [PubMed]
- Mavčič, B.; Babnik, B.; Iglič, A.; Kandušer, M.; Slivnik, T.; Kralj-Iglič, V. Shape transformation of giant phospholipid vesicles at high concentrations of C12E8. Bioelectrochemistry 2004, 63, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Inaoka, Y.; Yamazaki, M. Vesicle fission of giant unilamellar vesicles of liquid-ordered-phase membranes induced by amphiphiles with a single long hydrocarbon chain. Langmuir 2007, 23, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Giger, K.; Lamberson, E.R.; Hovis, J.S. Formation of complex three-dimensional structures in supported lipid bilayers. Langmuir 2008, 25, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Staykova, M.; Arroyo, M.; Rahimi, M.; Stone, H.A. Confined bilayers passively regulate shape and stress. Phys. Rev. Lett. 2013, 110, 028101. [Google Scholar] [CrossRef] [PubMed]
- Cambrea, L.R.; Hovis, J.S. Formation of three-dimensional structures in supported lipid bilayers. Biophys. J. 2007, 92, 3587–3594. [Google Scholar] [CrossRef] [PubMed]
- Seu, K.J.; Cambrea, L.R.; Everly, R.M.; Hovis, J.S. Influence of lipid chemistry on membrane fluidity: Tail and headgroup interactions. Biophys. J. 2006, 91, 3727–3735. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.K.; Jackman, J.A.; Kim, M.C.; Sut, T.N.; Cho, N.-J. Correlating membrane morphological responses with micellar aggregation behavior of capric acid and monocaprin. Langmuir 2017, 33, 2750–2759. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, L.M.; Yoon, B.K.; Jackman, J.A.; Knoll, W.; Weiss, P.S.; Cho, N.-J. Understanding how sterols regulate membrane remodeling in supported lipid bilayers. Langmuir 2017, 33, 14756–14765. [Google Scholar] [CrossRef] [PubMed]
- Lee, A. Prevention of Helicobacter pylori infection. Scand. J. Gastroenterol. 1996, 31 (Suppl. 215), 11–15. [Google Scholar] [CrossRef]
- Pattison, C.; Combs, M.; Marshall, B. Helicobacter pylori and peptic ulcer disease: Evolution to revolution to resolution. Am. J. Roentgenol. 1997, 168, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Amieva, M.; Peek, R.M. Pathobiology of Helicobacter pylori–induced gastric cancer. Gastroenterology 2016, 150, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Molina-Infante, J.; Lucendo, A.; Angueira, T.; Rodriguez-Tellez, M.; Perez-Aisa, A.; Balboa, A.; Barrio, J.; Martin-Noguerol, E.; Gomez-Rodriguez, B.; Botargues-Bote, J. Optimised empiric triple and concomitant therapy for Helicobacter pylori eradication in clinical practice: The OPTRICON study. Aliment. Pharmacol. Therap. 2015, 41, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Fallone, C.A.; Chiba, N.; van Zanten, S.V.; Fischbach, L.; Gisbert, J.P.; Hunt, R.H.; Jones, N.L.; Render, C.; Leontiadis, G.I.; Moayyedi, P. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology 2016, 151, 51.e14–69.e14. [Google Scholar] [CrossRef] [PubMed]
- Chey, W.D.; Leontiadis, G.I.; Howden, C.W.; Moss, S.F. ACG clinical guideline: Treatment of Helicobacter pylori infection. Am. J. Gastroenterol. 2017, 112, 212. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.; Michel, V.; Matos, A.A.; Carvalho, P.; Oliveira, M.J.; Ferreira, R.M.; Dillies, M.-A.; Huerre, M.; Seruca, R.; Figueiredo, C. Docosahexaenoic acid inhibits Helicobacter pylori growth in vitro and mice gastric mucosa colonization. PLoS ONE 2012, 7, e35072. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.; Michel, V.; Osório, H.; El Ghachi, M.; Bonis, M.; Boneca, I.G.; De Reuse, H.; Matos, A.A.; Lenormand, P.; Seruca, R. Crosstalk between Helicobacter pylori and gastric epithelial cells is impaired by docosahexaenoic acid. PLoS ONE 2013, 8, e60657. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Lim, J.W.; Kim, J.M.; Kim, H. Anti-inflammatory mechanism of polyunsaturated fatty acids in Helicobacter pylori-infected gastric epithelial cells. Mediat. Inflamm. 2014, 2014, 128919. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.W.; Lee, S.W. The antibacterial effect of fatty acids on Helicobacter pylori infection. Korean J. Intern. Med. 2016, 31, 30. [Google Scholar] [CrossRef] [PubMed]
- Obonyo, M.; Zhang, L.; Thamphiwatana, S.; Pornpattananangkul, D.; Fu, V.; Zhang, L. Antibacterial activities of liposomal linolenic acids against antibiotic-resistant Helicobacter pylori. Mol. Pharm. 2012, 9, 2677–2685. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.W.; Thamphiwatana, S.; Zhang, L.; Obonyo, M. Mechanism of antibacterial activity of liposomal linolenic acid against Helicobacter pylori. PLoS ONE 2015, 10, e0116519. [Google Scholar] [CrossRef] [PubMed]
- Thamphiwatana, S.; Gao, W.; Obonyo, M.; Zhang, L. In vivo treatment of Helicobacter pylori infection with liposomal linolenic acid reduces colonization and ameliorates inflammation. Proc. Natl. Acad. Sci. USA 2014, 111, 17600–17605. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-X.; Shi, S.; Rong, L.; Feng, M.-Q.; Zhong, L. The impact of liposomal linolenic acid on gastrointestinal microbiota in mice. Int. J. Nanomed. 2018, 13, 1399. [Google Scholar] [CrossRef] [PubMed]
- Seabra, C.L.; Nunes, C.; Gomez-Lazaro, M.; Correia, M.; Machado, J.C.; Gonçalves, I.C.; Reis, C.A.; Reis, S.; Martins, M.C.L. Docosahexaenoic acid loaded lipid nanoparticles with bactericidal activity against Helicobacter pylori. Int. J. Pharm. 2017, 519, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Tsai, T.-H.; Chuang, L.-T.; Li, Y.-Y.; Zouboulis, C.C.; Tsai, P.-J. Anti-bacterial and anti-inflammatory properties of capric acid against Propionibacterium acnes: A comparative study with lauric acid. J. Dermatol. Sci. 2014, 73, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Pornpattananangkul, D.; Nakatsuji, T.; Chan, M.; Carson, D.; Huang, C.-M.; Zhang, L. The antimicrobial activity of liposomal lauric acids against Propionibacterium acnes. Biomaterials 2009, 30, 6035–6040. [Google Scholar] [CrossRef] [PubMed]
- Pornpattananangkul, D.; Fu, V.; Thamphiwatana, S.; Zhang, L.; Chen, M.; Vecchio, J.; Gao, W.; Huang, C.M.; Zhang, L. In vivo treatment of Propionibacterium acnes infection with liposomal lauric acids. Adv. Healthc. Mater. 2013, 2, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.-Q.-M.; Hsieh, M.-F.; Chang, K.-L.; Pho, Q.-H.; Nguyen, V.-C.; Cheng, C.-Y.; Huang, C.-M. Bactericidal effect of lauric acid-loaded PCL-PEG-PCL nano-sized micelles on skin commensal Propionibacterium acnes. Polymers 2016, 8, 321. [Google Scholar] [CrossRef]
- Silva, E.L.; de Carvalho, M.; Santos, S.; Ferreira, L. Solid lipid nanoparticles loaded with retinoic acid and lauric acid as an alternative for topical treatment of acne vulgaris. J. Nanosci. Nanotechnol. 2014, 14, 1–8. [Google Scholar] [CrossRef]
- Chen, C.-H.; Wang, Y.; Nakatsuji, T.; Liu, Y.-T.; Zouboulis, C.; Gallo, R.L.; Zhang, L.; Hsieh, M.-F.; Huang, C.-M. An innate bactericidal oleic acid effective against skin infection of methicillin-resistant Staphylococcus aureus: A therapy concordant with evolutionary medicine. J. Microbiol. Biotechnol. 2011, 21, 391–399. [Google Scholar] [PubMed]
- Zhang, H.; Cui, Y.; Zhu, S.; Feng, F.; Zheng, X. Characterization and antimicrobial activity of a pharmaceutical microemulsion. Int. J. Pharm. 2010, 395, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Jackman, J.A.; Yoon, B.K.; Li, D.; Cho, N.J. Nanotechnology formulations for antibacterial free fatty acids and monoglycerides. Molecules 2016, 21, 305. [Google Scholar] [CrossRef] [PubMed]








| Bacteria (*) | Fatty Acid [FAs]/Monoglycerides [MGs] † | Key Findings | Ref. |
|---|---|---|---|
| B. megaterium (+) B. mycoides (+) B. subtilis (+) Bacillus sp. (+) Strep. faecium (+) Strep. lactis (+) Staphylococcus sp. (+) Micrococcus sp. (+) M. lysodeikticus (+) Cl. butyricum (+) Cl. sporogenes (+) Cl. welchii (+) | [FAs ‡]: C8:0, C10:0, C12:0, C14:0, C16:0, C18:0, C18:1, trans-C18:1, C18:2, C18:3 |
| [50] |
| Pneumococci (+) Streptococcus group A (+) Streptococcus beta-hemolytic non-A (+) Corynebacterium sp. (+) N. asteroides (+) Micrococcus sp. (+) S. aureus (+) S. epidermidis (+) Streptococcus group D (+) | [FAs]: C6:0, C8:0, C10:0, C12:0, C14:0, C16:0, C18:0, C14:1, C16:1, C18:1, trans-C18:1, C18:2, trans-C18:2, C18:3, C20:4 [MGs §]: C10:0, C12:0 |
| [14] |
| Strep. faecalis (+) Strep. pyogenes (+) S. aureus (+) Corynebacterium sp. (+) N. asteroides (+) | [FAs]: C11:0, C12:0, C13:0 [MGs]: C11:0, C12:0, C13:0 |
| [15] |
| M. smegmatis (+) | [FAs]: C10:0, C12:0, C14:0, C16:0, C18:0, C20:0, C16:1, C18:1, C18:2, C20:4 |
| [60] |
| S. aureus (+) L. acidophilus (+) B. megaterium (+) H. influenzae (−) N. gonorrhoeae (−) E. coli (−) | [FA]: C20:4 |
| [27] |
| L. monocytogenes (+) | [FAs]: C12:0, C14:0, C16:0, C18:0, C18:1, C18:2, C18:3 [MGs] C12:0, C14:0, C16:0 |
| [28] |
| B. larvae (+) | [FAs]: C10:0, C11:0, C12:0, C13:0, C14:1, C16:1, C18:2, etc. |
| [52] |
| H. pylori (−) | [FAs]: C4:0, C5:0, C6:0, C7:0, C8:0, C9:0, C10:0, C11:0, C12:0, C13:0, C14:0, C15:0, C16:0, C17:0, C12:1 [MGs]: C4:0, C5:0, C6:0, C7:0, C8:0, C9:0, C10:0, C11:0, C12:0, C13:0, C14:0, C15:0, C16:0, C17:0, C12:1 |
| [62] |
| C. trachomatis (−) | [FAs]: C8:0, C10:0, C12:0, C14:0, C16:1, C18:1 [MGs]: C8:0, C10:0, C12:0, C16:1, C18:1 |
| [29] |
| N. gonorrhoeae (−) | [FAs]: C8:0, C10:0, C12:0, C14:0, C16:1, C18:1 [MGs]: C8:0, C10:0, C12:0, C14:0, C16:1, C18:1 |
| [63] |
| Streptococcus group A (+) Streptococcus group B (+) S. aureus (+) | [FAs]: C8:0, C10:0, C12:0, C14:0, C16:1, C18:1 [MGs]: C8:0, C10:0, C12:0, C14:0, C16:1, C18:1 |
| [30] |
| H. pylori (−) E. coli (−) Salmonella spp. (−) | [FAs]: C8:0, C10:0, C12:0, C14:0, C16:1, C18:1 [MGs]: C8:0, C10:0, C12:0, C14:0, C16:1, C18:1 |
| [64] |
| H. pylori (−) | [FAs]: C4:0, C6:0, C8:0, C10:0, C12:0, C14:0, C16:0, C14:1, C16:1, C18:1, C18:2, C18:3 [MGs]: C12:0, C14:0, C16:0 |
| [54] |
| E. coli (−) | [FAs]: C2:0, C3:0, C4:0, C5:0, C6:0, C8:0, C10:0, C12:0, C14:0, C16:0, C18:0, C18:1, C18:2 |
| [65] |
| S. enteritidis (−) S. infantis (−) S. typhimurium (−) | [FAs]: C2:0, C3:0, C4:0, C5:0, C6:0, C8:0, C10:0, C12:0, C14:0, C16:0, C18:0, C18:1, C18:2 |
| [66] |
| S. aureus (+) Methicillin-Susceptible Staphylococcus aureus (MSSA) (+) Methicillin-Resistant Staphylococcus aureus (MRSA) (+) | [FAs]: C8:0, C10:0, C12:0, C14:0, C16:0, C18:0 |
| [19] |
| C. perfringens (+) | [FAs]: C2:0, C3:0, C4:0, C5:0, C6:0, C8:0, C10:0, C12:0, C14:0, C16:0, C18:0, C18:1, C18:2 |
| [31] |
| L. garvieae (+) V. harveyi (−) V. anguillarium (−) V. alginocolyticus (−) | [FAs]: C15:0, C16:0, C17:0, C18:0, C22:0, C18:1, C18:4, C20:4, C20:5, C22:4, C22:5 |
| [67] |
| B. cereus (+) S. aureus (+) E. coli (−) V. parahaemolyticus (−) S. typhimurium (−) S. enteritidis (−) | [FA]: C18:3 [MGs]: C12:0, C14:0 |
| [68] |
| Strep. iniae (+) E. ictaluri (−) E. tarda (−) Y. ruckeri (−) | [FA]: C8:0 [MG]: C8:0 |
| [69] |
| P. acnes (+) S. aureus (+) S. epidermidis (+) | [FA]: C12:0 |
| [20] |
| S. aureus (+) B. subtilis (+) E. coli (−) | [MG]: C12:0 |
| [70] |
| S. aureus (+) Strep. pyogenes (+) | [FA]: C12:0 [MG]: C12:0 |
| [21] |
| S. aureus (+) B. cereus (+) E. coli (−) P. aeruginosa (−) | [MGs]: C11:0, C11:1 |
| [71] |
| C. sakazakii (−) C. malonaticus (−) | [FAs]: C6:2, C8:0, C10:0, C12:0 [MGs]: C6:2, C8:0, C10:0, C12:0 |
| [72] |
| Platform | Technique | Technical Points | |
|---|---|---|---|
| Biological Approaches | Growth Inhibition Assays | Minimum inhibitory concentration (MIC) |
|
| Infectivity Assays | Minimum bactericidal concentration (MBC) |
| |
| Electron Microscopy | Transmission electron microscopy (TEM) Scanning electron microscopy (SEM) |
| |
| Biophysical Approaches | Solution-Phase Liposomes (SUVs and LUVs) | Dynamic light scattering (DLS) Electron microscopy |
|
| Giant Unilamellar Vesicle (GUV) | Phase-contrast microscopy Fluorescence microscopy |
| |
| Supported Lipid Bilayer (SLB) | Quartz crystal microbalance-dissipation (QCM-D) Fluorescence microscopy Fluorescence recovery after photobleaching (FRAP) |
|
| Bacteria | Fatty Acids * (Number of Carbon Atoms in Alkyl Chain:Number of Double Bonds) | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C10:0 | C12:0 | C14:0 | C16:0 | C18:0 | C18:1 | C18:2 | C18:3 | ||
| B. megaterium | 1.0 mM | 0.15 mM | 0.15 mM | 0.3 mM | 0.4 mM | 0.05 mM | 0.02 mM | 0.02 mM | [50] |
| Pneumococci | 1.45 mM | 0.062 mM | 0.218 mM | 0.48 mM | NI † | NI | 0.044 mM | 0.179 mM | [14] |
| Streptococcus group A | 1.45 mM | 0.124 mM | 0.547 mM | 3.9 mM | NI | 1.77 mM | 0.089 mM | 0.35 mM | |
| Streptococcus group D | 5.8 mM | 2.49 mM | 4.37 mM | NI | NI | NI | NI | NI | |
| Streptococcus beta-hemolyticnon-A | 2.9 mM | 0.249 mM | 2.18 mM | 3.9 mM | NI | NI | 0.089 mM | 0.35 mM | |
| Micrococcus sp. | 2.9 mM | 0.624 mM | 0.547 mM | 1.9 mM | NI | NI | 0.089 mM | 0.488 mM | |
| Corynebacterium sp. | 1.45 mM | 0.124 mM | 0.437 mM | 1.9 mM | NI | NI | 0.044 mM | 0.179 mM | |
| -- | 31 µg/mL | -- | -- | -- | -- | -- | -- | [15] | |
| N. asteroides | 1.45 mM | 0.124 mM | 0.547 mM | NI | NI | NI | 0.089 mM | 0.448 mM | [14] |
| -- | 62 µg/mL | -- | -- | -- | -- | -- | -- | [15] | |
| S. epidermidis | 2.9 mM | 2.49 mM | 2.18 mM | 3.9 mM | NI | NI | NI | NI | [14] |
| -- | 3.9 µg/mL | -- | -- | -- | -- | -- | -- | [20] | |
| S. aureus | 2.9 mM | 2.49 mM | 4.37 mM | NI | NI | NI | NI | 1.79 mM | [14] |
| -- | 500 µg/mL | -- | -- | -- | -- | -- | -- | [15] | |
| -- | 0.97 µg/mL | -- | -- | -- | -- | -- | -- | [20] | |
| MSSA | 800 µg/mL | 400 µg/mL | 1600 µg/mL | >1600 µg/mL | >1600 µg/mL | -- | -- | -- | [19] |
| MRSA | 800 µg/mL | 400 µg/mL | 1600 µg/mL | >1600 µg/mL | >1600 µg/mL | -- | -- | -- | |
| Strep. faecalis | -- | 500 µg/mL | -- | -- | -- | -- | -- | -- | [15] |
| Strep. pyogenes | -- | 62 µg/mL | -- | -- | -- | -- | -- | -- | |
| P. acnes | -- | 3.9 µg/mL | -- | -- | -- | -- | -- | -- | [20] |
| Bacteria | Monoglycerides * (Number of Carbon Atoms in Alkyl Chain:Number of Double Bonds) | Ref. | ||
|---|---|---|---|---|
| C10:0 | C12:0 | C13:0 | ||
| Pneumococci | 0.1 mM | 0.09 mM | -- | [14] |
| Streptococcus group A | 0.2 mM | 0.045 mM | -- | |
| Streptococcus group D | 2.0 mM | NI † | -- | |
| Streptococcus beta-hemolyticnon-A | 0.2 mM | 0.09 mM | ||
| Micrococcus sp. | 0.1 mM | 0.09 mM | -- | |
| S. epidermidis | 1.0 mM | 0.09 mM | -- | |
| Corynebacterium sp. | 0.2 mM | 0.045 mM | -- | |
| -- | 16 µg/mL | NI | [15] | |
| N. asteroides | 0.5 mM | 0.09 mM | -- | [14] |
| -- | 16 µg/mL | 125 µg/mL | [15] | |
| S. aureus | 1.0 mM | 0.09 mM | -- | [14] |
| -- | 250 µg/mL | NI | [15] | |
| Strep. faecalis | -- | NI | NI | |
| Strep. pyogenes | -- | 8 µg/mL | 62 µg/mL | |
| Antimicrobial Lipid | Bacteria * | Technique † | Key Observations | Ref. |
|---|---|---|---|---|
| Oleic acid | Streptococcus group A (+) | TEM |
| [26] |
| Arachidonic acid | N. gonorrhoeae (−) S. aureus (+) | TEM |
| [27] |
| Linolenic acid Glycerol Monolaurate (GML) | L. monocytogenes (+) | TEM |
| [28] |
| Monocaprin | C. trachomatis (−) | TEM |
| [29] |
| Monocaprin | Streptococcus group B (+) | TEM SEM |
| [30] |
| Lauric acid (LA) | C. perfringens (+) | TEM |
| [31] |
| Eicosapentaenoic acid (EPA) | S. aureus (+) P. aeruginosa (−) | SEM |
| [32] |
| Liposome (Composition) | Fatty Acid/Anion | Techniques | Key Observations | Ref. |
|---|---|---|---|---|
| SUVs (POPC) | Oleate | Electron microscopy Dynamic light scattering UV/VIS spectrophotometry |
| [33] |
| SUVs (POPC) | Oleate | Cryo-TEM UV/VIS spectrophotometry |
| [34] |
| SUVs (POPC) | Oleate | Cryo-TEM |
| [35] |
| SUVs (POPC) | Oleate | Dynamic light scattering Optical density |
| [36] |
| SUVs, LUVs (Egg PC) | Oleate | Gel filtration chromatography combined with dynamic light scattering (DLS) UV/VIS spectrophotometry |
| [37] |
| SUVs, LUVs (Egg PC) | Oleate | Electron microscopy Dynamic light scattering Gel exclusion chromatography |
| [38] |
| SUVs, LUVs (Egg PC) | Oleate | Gel exclusion chromatography |
| [39] |
| SUVs (DMPC, POPC) | Capric acid, Oleic acid, Linoleic acid | Electron microscopy Dynamic light scattering Light microscopy UV/VIS spectrophotometry |
| [40] |
| SLB Composition | Single-Chain Amphiphiles | Techniques | Key Observations | Ref. |
|---|---|---|---|---|
| DOPC/PA | Lysophosphatidylcholine (LPC) | Fluorescence microscopy FRAP |
| [128] |
| Egg PC | LPC Lysophosphatidylethanolamine (LPE) | FRAP ATR-FTIR |
| [131] |
| POPC | Docosahexaenoic acid (DHA) | QCM-D Fluorescence microscopy |
| [43] |
| POPC, POPC/PS, POPC/PI | Docosahexaenoic acid (DHA) | QCM-D |
| [44] |
| DOPC | LA GML SDS | QCM-D Fluorescence microscopy |
| [109] |
| DOPC | Capric acid Monocaprin | QCM-D Fluorescence microscopy FRAP |
| [132] |
| DOPC/Cholesterol | LA GML SDS | QCMD |
| [133] |
| Bacterial lipid extracts (E. coli) | Monocaprylate | QCM-D AFM |
| [45] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, B.K.; Jackman, J.A.; Valle-González, E.R.; Cho, N.-J. Antibacterial Free Fatty Acids and Monoglycerides: Biological Activities, Experimental Testing, and Therapeutic Applications. Int. J. Mol. Sci. 2018, 19, 1114. https://doi.org/10.3390/ijms19041114
Yoon BK, Jackman JA, Valle-González ER, Cho N-J. Antibacterial Free Fatty Acids and Monoglycerides: Biological Activities, Experimental Testing, and Therapeutic Applications. International Journal of Molecular Sciences. 2018; 19(4):1114. https://doi.org/10.3390/ijms19041114
Chicago/Turabian StyleYoon, Bo Kyeong, Joshua A. Jackman, Elba R. Valle-González, and Nam-Joon Cho. 2018. "Antibacterial Free Fatty Acids and Monoglycerides: Biological Activities, Experimental Testing, and Therapeutic Applications" International Journal of Molecular Sciences 19, no. 4: 1114. https://doi.org/10.3390/ijms19041114