Amniotic Fluid Stem Cells: A Novel Source for Modeling of Human Genetic Diseases
Abstract
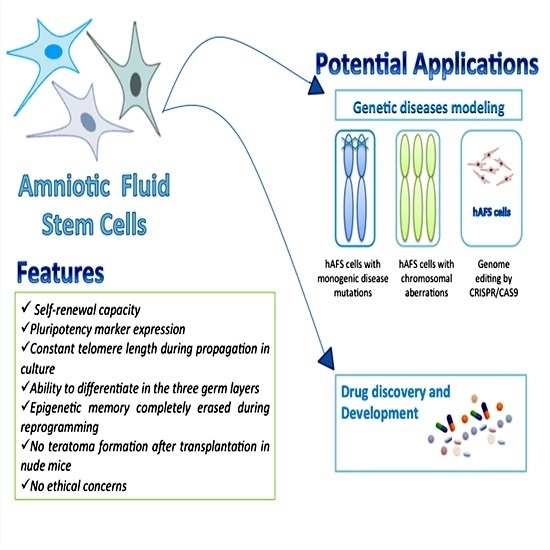
:1. Background
2. AFS Cells: Features and Properties
3. Are AFS Cells Pluripotent Stem Cells?
4. Reprogramming AFS Cells from Human Disease
5. AFS Cells and Drug Testing
6. Future Perspectives: AFS Cells for the Study of Trans-Generational Effects of Epigenetic Alterations
7. Conclusions
- (1)
- AFS cells can be easily collected from women undergoing amniocentesis, a prenatal diagnostic tool currently used in Western countries in a growing number of pregnancies, mostly due to the increased cases characterized by advanced maternal age caused by social and cultural factors. The recent improvements in the first trimester non-invasive tests for prenatal diagnosis will likely reduce the total number of amniocentesis, but will increase the rate of genetically abnormal pregnancies, thus providing a large number of samples to be used for the modeling of specific genetic diseases;
- (2)
- Unlike iPS, AFS cells will provide the opportunity to also investigate diseases that are lethal during pregnancy. Moreover, AFS cells are less prone to aging dependent genetic and epigenetic modifications as compared to fibroblasts, commonly used for the generation of iPS, thus providing a more natural model for the study of the disease;
- (3)
- AFS cells can be easily reprogrammed by using both viral and non-viral methods, making this process very efficient. Moreover, the use of non-viral methods will likely reduce the cost of the reprogramming procedure as compared to the use of iPS, which also requires the availability of specific facilities for the use of viral vectors.
Author Contributions
Conflicts of Interest
References
- Zhu, H.; Lensch, M.W.; Cahan, P.; Daley, G.Q. Investigating monogenic and complex diseases with pluripotent stem cells. Nat. Rev. Genet. 2011, 12, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Kobold, S.; Guhr, A.; Kurtz, A.; Loser, P. Human embryonic and induced pluripotent stem cell research trends: Complementation and diversification of the field. Stem Cell Rep. 2015, 4, 914–925. [Google Scholar] [CrossRef] [PubMed]
- Park, I.H.; Zhao, R.; West, J.A.; Yabuuchi, A.; Huo, H.; Ince, T.A.; Lerou, P.H.; Lensch, M.W.; Daley, G.Q. Reprogramming of human somatic cells to pluripotency with defined factors. Nature 2008, 451, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, B.; Li, Y.; Han, L.; Kaplan, A.D.; Ao, Y.; Kalra, S.; Bett, G.C.; Rasmusson, R.L.; Denning, C.; Yang, L. Modeling and study of the mechanism of dilated cardiomyopathy using induced pluripotent stem cells derived from individuals with duchenne muscular dystrophy. Dis. Model. Mech. 2015, 8, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Juopperi, T.A.; Kim, W.R.; Chiang, C.H.; Yu, H.; Margolis, R.L.; Ross, C.A.; Ming, G.L.; Song, H. Astrocytes generated from patient induced pluripotent stem cells recapitulate features of huntington’s disease patient cells. Mol. Brain 2012, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Tiscornia, G.; Vivas, E.L.; Matalonga, L.; Berniakovich, I.; Barragan Monasterio, M.; Eguizabal, C.; Gort, L.; Gonzalez, F.; Ortiz Mellet, C.; Garcia Fernandez, J.M.; et al. Neuronopathic gaucher’s disease: Induced pluripotent stem cells for disease modelling and testing chaperone activity of small compounds. Hum. Mol. Genet. 2013, 22, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Urbach, A.; Bar-Nur, O.; Daley, G.Q.; Benvenisty, N. Differential modeling of fragile x syndrome by human embryonic stem cells and induced pluripotent stem cells. Cell Stem Cell 2010, 6, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Loh, Y.H.; McLoughlin, E.M.; Huang, J.; Park, I.H.; Miller, J.D.; Huo, H.; Okuka, M.; Dos Reis, R.M.; Loewer, S.; et al. Telomere elongation in induced pluripotent stem cells from dyskeratosis congenita patients. Nature 2010, 464, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Moretti, A.; Bellin, M.; Welling, A.; Jung, C.B.; Lam, J.T.; Bott-Flugel, L.; Dorn, T.; Goedel, A.; Hohnke, C.; Hofmann, F.; et al. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N. Engl. J. Med. 2010, 363, 1397–1409. [Google Scholar] [CrossRef] [PubMed]
- Marchetto, M.C.; Carromeu, C.; Acab, A.; Yu, D.; Yeo, G.W.; Mu, Y.; Chen, G.; Gage, F.H.; Muotri, A.R. A model for neural development and treatment of rett syndrome using human induced pluripotent stem cells. Cell 2010, 143, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.N.; Byers, B.; Cord, B.; Shcheglovitov, A.; Byrne, J.; Gujar, P.; Kee, K.; Schule, B.; Dolmetsch, R.E.; Langston, W.; et al. LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell 2011, 8, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Doi, A.; Wen, B.; Ng, K.; Zhao, R.; Cahan, P.; Kim, J.; Aryee, M.J.; Ji, H.; Ehrlich, L.I.; et al. Epigenetic memory in induced pluripotent stem cells. Nature 2010, 467, 285–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gore, A.; Li, Z.; Fung, H.L.; Young, J.E.; Agarwal, S.; Antosiewicz-Bourget, J.; Canto, I.; Giorgetti, A.; Israel, M.A.; Kiskinis, E.; et al. Somatic coding mutations in human induced pluripotent stem cells. Nature 2011, 471, 63–67. [Google Scholar] [CrossRef] [PubMed]
- De Coppi, P.; Bartsch, G., Jr.; Siddiqui, M.M.; Xu, T.; Santos, C.C.; Perin, L.; Mostoslavsky, G.; Serre, A.C.; Snyder, E.Y.; Yoo, J.J.; et al. Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol. 2007, 25, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Pipino, C.; Pierdomenico, L.; di Tomo, P.; di Giuseppe, F.; Cianci, E.; D’Alimonte, I.; Morabito, C.; Centurione, L.; Antonucci, I.; Mariggio, M.A.; et al. Molecular and phenotypic characterization of human amniotic fluid-derived cells: A morphological and proteomic approach. Stem Cells Dev. 2015, 24, 1415–1428. [Google Scholar] [CrossRef] [PubMed]
- Di Trapani, M.; Bassi, G.; Fontana, E.; Giacomello, L.; Pozzobon, M.; Guillot, P.V.; de Coppi, P.; Krampera, M. Immune regulatory properties of CD117pos amniotic fluid stem cells vary according to gestational age. Stem Cells Dev. 2015, 24, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Savickiene, J.; Treigyte, G.; Baronaite, S.; Valiuliene, G.; Kaupinis, A.; Valius, M.; Arlauskiene, A.; Navakauskiene, R. Human amniotic fluid mesenchymal stem cells from second- and third-trimester amniocentesis: Differentiation potential, molecular signature, and proteome analysis. Stem Cells Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Prusa, A.R.; Hengstschlager, M. Amniotic fluid cells and human stem cell research: A new connection. Med. Sci. Monit. 2002, 8, RA253–RA257. [Google Scholar] [PubMed]
- Antonucci, I.; Pantalone, A.; Tete, S.; Salini, V.; Borlongan, C.V.; Hess, D.; Stuppia, L. Amniotic fluid stem cells: A promising therapeutic resource for cell-based regenerative therapy. Curr. Pharm. Des. 2012, 18, 1846–1863. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, I.; Stuppia, L.; Kaneko, Y.; Yu, S.; Tajiri, N.; Bae, E.C.; Chheda, S.H.; Weinbren, N.L.; Borlongan, C.V. Amniotic fluid as a rich source of mesenchymal stromal cells for transplantation therapy. Cell Transplant. 2011, 20, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Prusa, A.R.; Marton, E.; Rosner, M.; Bernaschek, G.; Hengstschlager, M. Oct-4-expressing cells in human amniotic fluid: A new source for stem cell research? Hum. Reprod. 2003, 18, 1489–1493. [Google Scholar] [CrossRef] [PubMed]
- In’t Anker, P.S.; Scherjon, S.A.; Kleijburg-van der Keur, C.; Noort, W.A.; Claas, F.H.; Willemze, R.; Fibbe, W.E.; Kanhai, H.H. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood 2003, 102, 1548–1549. [Google Scholar] [CrossRef] [PubMed]
- Cananzi, M.; de Coppi, P. CD117+ amniotic fluid stem cells: State of the art and future perspectives. Organogenesis 2012, 8, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, I.; Iezzi, I.; Morizio, E.; Mastrangelo, F.; Pantalone, A.; Mattioli-Belmonte, M.; Gigante, A.; Salini, V.; Calabrese, G.; Tete, S.; et al. Isolation of osteogenic progenitors from human amniotic fluid using a single step culture protocol. BMC Biotechnol. 2009, 9. [Google Scholar] [CrossRef] [PubMed]
- D’Alimonte, I.; Lannutti, A.; Pipino, C.; di Tomo, P.; Pierdomenico, L.; Cianci, E.; Antonucci, I.; Marchisio, M.; Romano, M.; Stuppia, L.; et al. Wnt signaling behaves as a “master regulator” in the osteogenic and adipogenic commitment of human amniotic fluid mesenchymal stem cells. Stem Cell Rev. 2013, 9, 642–654. [Google Scholar] [CrossRef] [PubMed]
- Pipino, C.; Mukherjee, S.; David, A.L.; Blundell, M.P.; Shaw, S.W.; Sung, P.; Shangaris, P.; Waters, J.J.; Ellershaw, D.; Cavazzana, M.; et al. Trisomy 21 mid-trimester amniotic fluid induced pluripotent stem cells maintain genetic signatures during reprogramming: Implications for disease modeling and cryobanking. Cell Reprogram. 2014, 16, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Morabito, C.; D’Alimonte, I.; Pierdomenico, L.; Pipino, C.; Guarnieri, S.; Caprara, G.A.; Antonucci, I.; Ciccarelli, R.; Marchisio, M.; Pandolfi, A.; et al. Calcitonin-induced effects on amniotic fluid-derived mesenchymal stem cells. Cell. Physiol. Biochem. 2015, 36, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Ditadi, A.; de Coppi, P.; Picone, O.; Gautreau, L.; Smati, R.; Six, E.; Bonhomme, D.; Ezine, S.; Frydman, R.; Cavazzana-Calvo, M.; et al. Human and murine amniotic fluid c-kit+ lin- cells display hematopoietic activity. Blood 2009, 113, 3953–3960. [Google Scholar] [CrossRef] [PubMed]
- Grisafi, D.; Piccoli, M.; Pozzobon, M.; Ditadi, A.; Zaramella, P.; Chiandetti, L.; Zanon, G.F.; Atala, A.; Zacchello, F.; Scarpa, M.; et al. High transduction efficiency of human amniotic fluid stem cells mediated by adenovirus vectors. Stem Cells Dev. 2008, 17, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Pozzobon, M.; Piccoli, M.; Schiavo, A.A.; Atala, A.; de Coppi, P. Isolation of c-kit+ human amniotic fluid stem cells from second trimester. Methods Mol. Biol. 2013, 1035, 191–198. [Google Scholar] [PubMed]
- Fauza, D. Amniotic fluid and placental stem cells. Best Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 877–891. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.S.; Lee, J.L.; Chang, Y.J.; Hwang, S.M. Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum. Reprod. 2004, 19, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, I.; Pantalone, A.; De Amicis, D.; D’Onofrio, S.; Stuppia, L.; Palka, G.; Salini, V. Human amniotic fluid stem cells culture onto titanium screws: A new perspective for bone engineering. J. Biol. Regul. Homeost. Agents 2009, 23, 277–279. [Google Scholar] [PubMed]
- Kim, J.; Lee, Y.; Kim, H.; Hwang, K.J.; Kwon, H.C.; Kim, S.K.; Cho, D.J.; Kang, S.G.; You, J. Human amniotic fluid-derived stem cells have characteristics of multipotent stem cells. Cell Prolif. 2007, 40, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Davydova, D.A. Stem cells in human amniotic fluid. Izv. Akad. Nauk Ser. Biol. 2010, 517–526. [Google Scholar] [CrossRef]
- Antonucci, I.; di Pietro, R.; Alfonsi, M.; Centurione, M.A.; Centurione, L.; Sancilio, S.; Pelagatti, F.; D’Amico, M.A.; di Baldassarre, A.; Piattelli, A.; et al. Human second trimester amniotic fluid cells are able to create embryoid body-like structures in vitro and to show typical expression profiles of embryonic and primordial germ cells. Cell Transplant. 2014, 23, 1501–1515. [Google Scholar] [CrossRef] [PubMed]
- Walther, G.; Gekas, J.; Bertrand, O.F. Amniotic stem cells for cellular cardiomyoplasty: Promises and premises. Catheter. Cardiovasc. Interv. 2009, 73, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.S.; Moon, J.H.; Jun, E.K.; Kim, J.; Maeng, I.; Kim, J.S.; Lee, J.H.; Baik, C.S.; Kim, A.; Cho, K.S.; et al. Secretory profiles and wound healing effects of human amniotic fluid-derived mesenchymal stem cells. Stem Cells Dev. 2010, 19, 887–902. [Google Scholar] [CrossRef] [PubMed]
- Mirabella, T.; Cilli, M.; Carlone, S.; Cancedda, R.; Gentili, C. Amniotic liquid derived stem cells as reservoir of secreted angiogenic factors capable of stimulating neo-arteriogenesis in an ischemic model. Biomaterials 2011, 32, 3689–3699. [Google Scholar] [CrossRef] [PubMed]
- Zagoura, D.S.; Roubelakis, M.G.; Bitsika, V.; Trohatou, O.; Pappa, K.I.; Kapelouzou, A.; Antsaklis, A.; Anagnou, N.P. Therapeutic potential of a distinct population of human amniotic fluid mesenchymal stem cells and their secreted molecules in mice with acute hepatic failure. Gut 2012, 61, 894–906. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, N.; Acosta, S.; Glover, L.E.; Bickford, P.C.; Jacotte Simancas, A.; Yasuhara, T.; Date, I.; Solomita, M.A.; Antonucci, I.; Stuppia, L.; et al. Intravenous grafts of amniotic fluid-derived stem cells induce endogenous cell proliferation and attenuate behavioral deficits in ischemic stroke rats. PLoS ONE 2012, 7, e43779. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, N.; Acosta, S.; Portillo-Gonzales, G.S.; Aguirre, D.; Reyes, S.; Lozano, D.; Pabon, M.; Dela Pena, I.; Ji, X.; Yasuhara, T.; et al. Therapeutic outcomes of transplantation of amniotic fluid-derived stem cells in experimental ischemic stroke. Front. Cell. Neurosci. 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Bollini, S.; Cheung, K.K.; Riegler, J.; Dong, X.; Smart, N.; Ghionzoli, M.; Loukogeorgakis, S.P.; Maghsoudlou, P.; Dube, K.N.; Riley, P.R.; et al. Amniotic fluid stem cells are cardioprotective following acute myocardial infarction. Stem Cells Dev. 2011, 20, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- Dobreva, M.P.; Pereira, P.N.; Deprest, J.; Zwijsen, A. On the origin of amniotic stem cells: Of mice and men. Int. J. Dev. Biol. 2010, 54, 761–777. [Google Scholar] [CrossRef] [PubMed]
- Moschidou, D.; Drews, K.; Eddaoudi, A.; Adjaye, J.; de Coppi, P.; Guillot, P.V. Molecular signature of human amniotic fluid stem cells during fetal development. Curr. Stem Cell Res. Ther. 2013, 8, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Da Sacco, S.; de Filippo, R.E.; Perin, L. Amniotic fluid as a source of pluripotent and multipotent stem cells for organ regeneration. Curr. Opin. Organ. Transplant. 2011, 16, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Tsangaris, G.; Weitzdorfer, R.; Pollak, D.; Lubec, G.; Fountoulakis, M. The amniotic fluid cell proteome. Electrophoresis 2005, 26, 1168–1173. [Google Scholar] [CrossRef] [PubMed]
- Ferdaos, N.; Nathan, S.; Nordin, N. Prospective full-term-derived pluripotent amniotic fluid stem (AFS) cells. Med. J. Malays. 2008, 63, 75–76. [Google Scholar]
- Valli, A.; Rosner, M.; Fuchs, C.; Siegel, N.; Bishop, C.E.; Dolznig, H.; Madel, U.; Feichtinger, W.; Atala, A.; Hengstschlager, M. Embryoid body formation of human amniotic fluid stem cells depends on mtor. Oncogene 2010, 29, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Moschidou, D.; Mukherjee, S.; Blundell, M.P.; Drews, K.; Jones, G.N.; Abdulrazzak, H.; Nowakowska, B.; Phoolchund, A.; Lay, K.; Ramasamy, T.S.; et al. Valproic acid confers functional pluripotency to human amniotic fluid stem cells in a transgene-free approach. Mol. Ther. 2012, 20, 1953–1967. [Google Scholar] [CrossRef] [PubMed]
- Anchan, R.M.; Quaas, P.; Gerami-Naini, B.; Bartake, H.; Griffin, A.; Zhou, Y.; Day, D.; Eaton, J.L.; George, L.L.; Naber, C.; et al. Amniocytes can serve a dual function as a source of ips cells and feeder layers. Hum. Mol. Genet. 2011, 20, 962–974. [Google Scholar] [CrossRef] [PubMed]
- Galende, E.; Karakikes, I.; Edelmann, L.; Desnick, R.J.; Kerenyi, T.; Khoueiry, G.; Lafferty, J.; McGinn, J.T.; Brodman, M.; Fuster, V.; et al. Amniotic fluid cells are more efficiently reprogrammed to pluripotency than adult cells. Cell Reprogram. 2010, 12, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Wolfrum, K.; Wang, Y.; Prigione, A.; Sperling, K.; Lehrach, H.; Adjaye, J. The large principle of cellular reprogramming: Lost, acquired and retained gene expression in foreskin and amniotic fluid-derived human ips cells. PLoS ONE 2010, 5, e13703. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zou, G.; Gao, Y.; Zhao, X.; Wang, H.; Huang, Q.; Jiang, L.; Guo, L.; Cheng, W. High efficiency of reprogramming CD34+ cells derived from human amniotic fluid into induced pluripotent stem cells with oct4. Stem Cells Dev. 2012, 21, 2322–2332. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Luo, Y.; Chen, X.; Li, Q.; Sun, X. Generation of human beta-thalassemia induced pluripotent stem cells from amniotic fluid cells using a single excisable lentiviral stem cell cassette. J. Reprod. Dev. 2012, 58, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Fan, Y.; Sun, X.; Yu, Y. Generation of induced pluripotent stem cells from human amniotic fluid cells by reprogramming with two factors in feeder-free conditions. J. Reprod. Dev. 2013, 59, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; di Bernardo, J.; Maiden, M.M.; Villa-Diaz, L.G.; Mabrouk, O.S.; Krebsbach, P.H.; O’Shea, K.S.; Kunisaki, S.M. Human transgene-free amniotic-fluid-derived induced pluripotent stem cells for autologous cell therapy. Stem Cells Dev. 2014, 23, 2613–2625. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.E.; Yang, Y.C.; Chen, S.M.; Su, H.L.; Huang, P.C.; Tsai, M.S.; Wang, T.H.; Tseng, C.P.; Hwang, S.M. Modeling neurogenesis impairment in down syndrome with induced pluripotent stem cells from trisomy 21 amniotic fluid cells. Exp. Cell Res. 2013, 319, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Rosner, M.; Schipany, K.; Hengstschlager, M. The decision on the “optimal” human pluripotent stem cell. Stem Cells Transl. Med. 2014, 3, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Eglen, R.; Reisine, T. Primary cells and stem cells in drug discovery: Emerging tools for high-throughput screening. Assay Drug Dev. Technol. 2011, 9, 108–124. [Google Scholar] [CrossRef] [PubMed]
- Stokes, W.S. Animals and the 3Rs in toxicology research and testing: The way forward. Hum. Exp. Toxicol. 2015, 34, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Ebert, A.D.; Yu, J.; Rose, F.F., Jr.; Mattis, V.B.; Lorson, C.L.; Thomson, J.A.; Svendsen, C.N. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature 2009, 457, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Itzhaki, I.; Maizels, L.; Huber, I.; Zwi-Dantsis, L.; Caspi, O.; Winterstern, A.; Feldman, O.; Gepstein, A.; Arbel, G.; Hammerman, H.; et al. Modelling the long qt syndrome with induced pluripotent stem cells. Nature 2011, 471, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Kolaja, K. Stem cells and stem cell-derived tissues and their use in safety assessment. J. Biol. Chem. 2014, 289, 4555–4561. [Google Scholar] [CrossRef] [PubMed]
- Heilker, R.; Traub, S.; Reinhardt, P.; Scholer, H.R.; Sterneckert, J. iPS cell derived neuronal cells for drug discovery. Trends Pharmacol. Sci. 2014, 35, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, N.; Qiang, R.; Wan, Q.; Qin, M.; Chen, S.; Wang, H. Human amniotic fluid stem cells possess the potential to differentiate into primordial follicle oocytes in vitro. Biol. Reprod. 2014, 90. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M. Cb2 receptors in reproduction. Br. J. Pharmacol. 2008, 153, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, D.Q.; Li, B.W.; Guan, L.D.; Yan, Z.F.; Li, Y.L.; Pei, X.T.; Yue, W.; Wang, M.; Lu, Y.P.; et al. Human amniotic fluid-derived stem cells can differentiate into hepatocyte-like cells in vitro and in vivo. Vitro Cell. Dev. Biol. Anim. 2011, 47, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Bollini, S.; Pozzobon, M.; Nobles, M.; Riegler, J.; Dong, X.; Piccoli, M.; Chiavegato, A.; Price, A.N.; Ghionzoli, M.; Cheung, K.K.; et al. In vitro and in vivo cardiomyogenic differentiation of amniotic fluid stem cells. Stem Cell Rev. 2011, 7, 364–380. [Google Scholar] [CrossRef] [PubMed]
- Maioli, M.; Contini, G.; Santaniello, S.; Bandiera, P.; Pigliaru, G.; Sanna, R.; Rinaldi, S.; Delitala, A.P.; Montella, A.; Bagella, L.; et al. Amniotic fluid stem cells morph into a cardiovascular lineage: Analysis of a chemically induced cardiac and vascular commitment. Drug Des. Dev. Ther. 2013, 7, 1063–1073. [Google Scholar]
- Gao, Y.; Connell, J.P.; Wadhwa, L.; Ruano, R.; Jacot, J.G. Amniotic fluid-derived stem cells demonstrated cardiogenic potential in indirect co-culture with human cardiac cells. Ann. Biomed. Eng. 2014, 42, 2490–2500. [Google Scholar] [CrossRef] [PubMed]
- Bossolasco, P.; Montemurro, T.; Cova, L.; Zangrossi, S.; Calzarossa, C.; Buiatiotis, S.; Soligo, D.; Bosari, S.; Silani, V.; Deliliers, G.L.; et al. Molecular and phenotypic characterization of human amniotic fluid cells and their differentiation potential. Cell Res. 2006, 16, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Maraldi, T.; Bertoni, L.; Riccio, M.; Zavatti, M.; Carnevale, G.; Resca, E.; Guida, M.; Beretti, F.; la Sala, G.B.; de Pol, A. Human amniotic fluid stem cells: Neural differentiation in vitro and in vivo. Cell Tissue Res. 2014, 357, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Xinaris, C.; Benedetti, V.; Novelli, R.; Abbate, M.; Rizzo, P.; Conti, S.; Tomasoni, S.; Corna, D.; Pozzobon, M.; Cavallotti, D.; et al. Functional human podocytes generated in organoids from amniotic fluid stem cells. J. Am. Soc. Nephrol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Ranga, A.; Gjorevski, N.; Lutolf, M.P. Drug discovery through stem cell-based organoid models. Adv. Drug Deliv. Rev. 2014, 69–70, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, A.Y.; Tung, Y.C.; Kuo, C.H.; Mosadegh, B.; Bedenis, R.; Pienta, K.J.; Takayama, S. Micro-ring structures stabilize microdroplets to enable long term spheroid culture in 384 hanging drop array plates. Biomed. Microdevices 2012, 14, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, R.; Vijftigschild, L.A.; Vonk, A.M.; Kruisselbrink, E.; de Winter-de Groot, K.M.; Janssens, H.M.; van der Ent, C.K.; Beekman, J.M. A bioassay using intestinal organoids to measure cftr modulators in human plasma. J. Cyst. Fibros. 2015, 14, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Stuppia, L.; Franzago, M.; Ballerini, P.; Gatta, V.; Antonucci, I. Epigenetics and male reproduction: The consequences of paternal lifestyle on fertility, embryo development, and children lifetime health. Clin. Epigenet. 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Rehan, V.K.; Liu, J.; Sakurai, R.; Torday, J.S. Perinatal nicotine-induced transgenerational asthma. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 305, L501–L507. [Google Scholar] [CrossRef] [PubMed]
- Manning, K.; Tor, M.; Poole, M.; Hong, Y.; Thompson, A.J.; King, G.J.; Giovannoni, J.J.; Seymour, G.B. A naturally occurring epigenetic mutation in a gene encoding an SBP-box transcription factor inhibits tomato fruit ripening. Nat. Genet. 2006, 38, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Pembrey, M.E.; Bygren, L.O.; Kaati, G.; Edvinsson, S.; Northstone, K.; Sjostrom, M.; Golding, J.; Team, A.S. Sex-specific, male-line transgenerational responses in humans. Eur. J. Hum. Genet. 2006, 14, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Yang, C.R.; Wei, Y.P.; Zhao, Z.A.; Hou, Y.; Schatten, H.; Sun, Q.Y. Paternally induced transgenerational inheritance of susceptibility to diabetes in mammals. Proc. Natl. Acad. Sci. USA 2014, 111, 1873–1878. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.K.; Guerrero-Bosagna, C.; Haque, M.; Nilsson, E.; Bhandari, R.; McCarrey, J.R. Environmentally induced transgenerational epigenetic reprogramming of primordial germ cells and the subsequent germ line. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Balmer, N.V.; Leist, M. Epigenetics and transcriptomics to detect adverse drug effects in model systems of human development. Basic Clin. Pharmacol. Toxicol. 2014, 115, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Vassoler, F.M.; White, S.L.; Schmidt, H.D.; Sadri-Vakili, G.; Pierce, R.C. Epigenetic inheritance of a cocaine-resistance phenotype. Nat. Neurosci. 2013, 16, 42–47. [Google Scholar] [CrossRef] [PubMed]


| Properties | ES | iPS | AFS |
|---|---|---|---|
| Naturally existing stem cells | Yes | No | Yes |
| Self renewal capacity | Yes | Yes | Yes |
| High proliferation efficiency | Yes | Yes | Yes |
| Pluripotent marker expression | Yes | Yes | Yes |
| Differentiation in three germ layers | Yes | Yes | Yes |
| Risk for teratoma formation | Yes | Yes | No |
| Ectopic oncogene expression | No | Yes | No |
| Immunological compatibility with recipient | Yes/No | Yes/No | Yes/No |
| Possessing prenatally lethal mutations | Yes | No | Yes/No |
| Disease-specific stem cells | Yes | Yes | Yes |
| Disease specific stem cells with known patient’s phenotype | No | Yes | Yes |
| Risk for chromosomal aberrations from the donor cells | No | Yes/No | No |
| Risk for aberrations acquired during reprogramming | No | Yes | No |
| Epigenetic deregulation | No | Yes | No |
| Studies on drug testing | Yes | Yes | Yes |
| Legal restrictions | Yes | No | No |
| Ethical concerns | Yes | No | No |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonucci, I.; Provenzano, M.; Rodrigues, M.; Pantalone, A.; Salini, V.; Ballerini, P.; Borlongan, C.V.; Stuppia, L. Amniotic Fluid Stem Cells: A Novel Source for Modeling of Human Genetic Diseases. Int. J. Mol. Sci. 2016, 17, 607. https://doi.org/10.3390/ijms17040607
Antonucci I, Provenzano M, Rodrigues M, Pantalone A, Salini V, Ballerini P, Borlongan CV, Stuppia L. Amniotic Fluid Stem Cells: A Novel Source for Modeling of Human Genetic Diseases. International Journal of Molecular Sciences. 2016; 17(4):607. https://doi.org/10.3390/ijms17040607
Chicago/Turabian StyleAntonucci, Ivana, Martina Provenzano, Melissa Rodrigues, Andrea Pantalone, Vincenzo Salini, Patrizia Ballerini, Cesar V. Borlongan, and Liborio Stuppia. 2016. "Amniotic Fluid Stem Cells: A Novel Source for Modeling of Human Genetic Diseases" International Journal of Molecular Sciences 17, no. 4: 607. https://doi.org/10.3390/ijms17040607