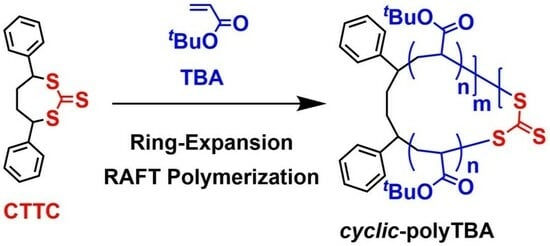
Development of Ring-Expansion RAFT Polymerization of tert-Butyl Acrylate with a Cyclic Trithiocarbonate Derivative toward the Facile Synthesis of Cyclic Polymers
Abstract
:1. Introduction
2. Results
2.1. Ring-Expansion RAFT Polymerization of TBA with CTTC
2.2. Characterization of the Polymers Obtained by RE-RAFT Polymerization with CTTC
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Methods
3.3. Ring-Expansion RAFT Polymerization of TBA with CTTC
3.4. Conventional RAFT Polymerization of TBA with DBTTC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Endo, K. Synthesis and Properties of Cyclic Polymers. Adv. Polym. Sci. 2008, 217, 121–183. [Google Scholar]
- Laurent, B.A.; Grayson, S.M. Synthetic approaches for the preparation of cyclic polymers. Chem. Soc. Rev. 2009, 38, 2202–2213. [Google Scholar] [CrossRef] [PubMed]
- Kricheldorf, H.R. Cyclic Polymers: Synthetic Strategies and Physical Properties. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 251–284. [Google Scholar] [CrossRef]
- Jia, Z.; Monteiro, M.J. Cyclic Polymers: Methods and Strategies. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 2085–2097. [Google Scholar] [CrossRef]
- Haque, F.M.; Grayson, S.M. The synthesis, properties and potential applications of cyclic polymers. Nat. Chem. 2020, 12, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ye, J.; Liu, S. Synthesis of Well-Defined Cyclic Poly(N-isopropylacrylamide) via Click Chemistry and Its Unique Thermal Phase Transition Behavior. Macromolecules 2007, 40, 9103–9110. [Google Scholar] [CrossRef]
- Qiu, X.-P.; Winnik, F.M. Effect of Topology on the Properties of Poly(N-isopropylacrylamide) in Water and in Bulk. Macromol. Symp. 2009, 278, 10–13. [Google Scholar] [CrossRef]
- Daneshyan, S.; Sodeifian, G. A new approach for synthesis of cyclic poly(N-isopropylacrylamide), for applying in biomaterial applications. Polym. Bull. 2024, 81, 929–949. [Google Scholar] [CrossRef]
- Adachi, K.; Honda, S.; Hayashi, S.; Tezuka, Y. ATRP-RCM Synthesis of Cyclic Diblock Copolymers. Macromolecules 2008, 41, 7898–7903. [Google Scholar] [CrossRef]
- Honda, S.; Yamamoto, T.; Tezuka, Y. Topology-Directed Control on Thermal Stability: Micelles Formed from Linear and Cyclized Amphiphilic Block Copolymers. J. Am. Chem. Soc. 2010, 132, 10251–10253. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Yamamoto, T.; Tezuka, Y. Tuneable enhancement of the salt and thermal stability of polymeric micelles by cyclized amphiphiles. Nat. Commun. 2013, 4, 1574. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.A.; Waymouth, R.M. Recent Progress on the Synthesis of Cyclic Polymers via Ring-Expansion Strategies. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 2892–2902. [Google Scholar] [CrossRef]
- Bielawski, C.W.; Benitez, D.; Grubbs, R.H. An “Endless” Route to Cyclic Polymers. Science 2002, 297, 2041–2044. [Google Scholar] [CrossRef] [PubMed]
- Culkin, D.A.; Jeong, W.; Csihony, S.; Gomez, E.D.; Balsara, N.P.; Hedrick, J.L.; Waymouth, R.M. Zwitterionic Polymerization of Lactide to Cyclic Poly(Lactide) by Using N-Heterocyclic Carbene Organocatalysts. Angew. Chem. Int. Ed. 2007, 46, 2627–2630. [Google Scholar] [CrossRef] [PubMed]
- Kaitz, J.A.; Diesendruck, C.E.; Moore, J.S. End Group Characterization of Poly(phthalaldehyde): Surprising Discovery of a Reversible, Cationic Macrocyclization Mechanism. J. Am. Chem. Soc. 2013, 135, 12755–12761. [Google Scholar] [CrossRef] [PubMed]
- Kammiyada, H.; Konishi, A.; Ouchi, M.; Sawamoto, M. Ring-Expansion Living Cationic Polymerization via Reversible Activation of a Hemiacetal Ester Bond. ACS Macro Lett. 2013, 2, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Chiefari, J.; Chong, Y.K.; Ercole, F.; Krstina, J.; Jeffery, J.; Le, T.P.L.; Mayadunne, R.T.A.; Meijs, G.F.; Moad, C.L.; Moad, G.; et al. Living Free-Radical Polymerization by Reversible Addition-Fragmentation Chain Transfer: The RAFT Process. Macromolecules 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- Perrier, S.; Takolpuckdee, P. Macromolecular Design via Reversible Addition-Fragmentation Chain Transfer (RAFT)/Xanthates (MADIX) Polymerization. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 5347–5393. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Radical addition–fragmentation chemistry in polymer synthesis. Polymer 2008, 49, 1079–1131. [Google Scholar] [CrossRef]
- Chen, M.; Zhong, M.; Johnson, J.A. Light-Controlled Radical Polymerization: Mechanisms, Methods, and Applications. Chem. Rev. 2016, 116, 10167–10211. [Google Scholar] [CrossRef] [PubMed]
- Minoda, M.; Otsubo, T.; Yamamoto, Y.; Zhao, J.; Honda, Y.; Tanaka, T.; Motoyanagi, J. The First Synthesis of Periodic and Alternating Glycopolymers by RAFT Polymerization: A Novel Synthetic Pathway for Glycosaminoglycan Mimics. Polymers 2019, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Motoyanagi, J.; Oguri, A.; Minoda, M. Synthesis of Well-Defined Alternating Copolymer Composed of Ethylmaleimide and Hydroxy-Functionalized Vinyl Ether by RAFT Polymerization and Their Thermoresponsive Properties. Polymers 2020, 12, 2255. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Fan, Z. Synthesis of Multiblock Polymer Containing Narrow Polydispersity Blocks. Macromol. Rapid Commun. 2006, 27, 57–62. [Google Scholar] [CrossRef]
- Lee, A.W.M.; Chan, W.H.; Wong, H.C. One Pot Phase Transfer Synthesis of Trithiocarbonates from Carbon Bisulphide and Alkyl Halides. Synth. Commun. 1988, 18, 1531–1536. [Google Scholar] [CrossRef]







| Reaction Time (h) | TBA Conversion (%) a | Mn, theory b (g mol−1) | Mn, cleaved c (g mol−1) | Mw/Mn c |
|---|---|---|---|---|
| 1 | 7 | 900 | 890 | 1.12 |
| 2 | 11 | 1400 | 1300 | 1.14 |
| 3 | 30 | 3800 | 3800 | 1.44 |
| 4 | 41 | 5300 | 5400 | 1.33 |
| 6 | 72 | 9200 | 9100 | 1.20 |
| 8 | 85 | 11,000 | 11,000 | 1.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motoyanagi, J.; Fujii, H.; Minoda, M. Development of Ring-Expansion RAFT Polymerization of tert-Butyl Acrylate with a Cyclic Trithiocarbonate Derivative toward the Facile Synthesis of Cyclic Polymers. Molecules 2024, 29, 1839. https://doi.org/10.3390/molecules29081839
Motoyanagi J, Fujii H, Minoda M. Development of Ring-Expansion RAFT Polymerization of tert-Butyl Acrylate with a Cyclic Trithiocarbonate Derivative toward the Facile Synthesis of Cyclic Polymers. Molecules. 2024; 29(8):1839. https://doi.org/10.3390/molecules29081839
Chicago/Turabian StyleMotoyanagi, Jin, Hiroki Fujii, and Masahiko Minoda. 2024. "Development of Ring-Expansion RAFT Polymerization of tert-Butyl Acrylate with a Cyclic Trithiocarbonate Derivative toward the Facile Synthesis of Cyclic Polymers" Molecules 29, no. 8: 1839. https://doi.org/10.3390/molecules29081839